FLIM-based Förster Resonance Energy Transfer (FRET)
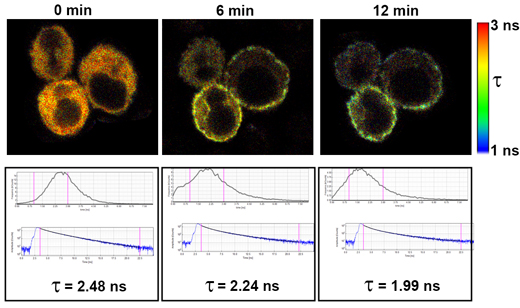
The fluorescence lifetime of Cerulean fluorescent protein was measured at various times after treatment with thapsigargin in Jurkat cells transfected to express Cerulean-STIM1 and Venus-Orai1. Thapsigargin causes a depletion of ER Ca2+ stores, resulting in STIM1 relocalization to associate with Orai1 on the plasma membrane and form CRAC Ca2+ channels.
As Cerulean-STIM1 associates with Venus-Orai1, FRET occurs between Cerulean (the donor) and Venus (the acceptor), which results in a shortening in the average fluorescence lifetime of Cerulean.
The images are fluorescence lifetime heatmaps based on the scale shown to the right. These images correspond to graphs of average lifetime, decay curves, and average Cerulean fluorescence lifetimes (τ).
Fluorescence lifetime imaging was performed on the Penn Vet Imaging Core’s Leica SP5 II inverted confocal/FLIM microscope with a 63x water immersion lens by scanning the sample with a 405 nm pulsed diode laser.
Images courtesy of Xiaohong Liu, Gordon Ruthel, and Bruce Freedman, Department of Pathobiology, UPenn School of Veterinary Medicine.