In the most severe form of male infertility, men do not make any measurable levels of sperm. This condition, called azoospermia, affects approximately 1 percent of the male population and is responsible for about a sixth of cases of male infertility.
Oftentimes men with azoospermia don’t know the underlying cause of their condition. But new research led by University of Pennsylvania scientists suggests that mutations in an X chromosome gene called TEX11 are responsible for a significant number of cases of infertility — an estimated 1 percent of cases of non-obstructive azoospermia.
 The paper is published in the journal EMBO Molecular Medicine. P. Jeremy Wang, a professor in the Department of Biomedical Sciences at Penn’s School of Veterinary Medicine, was the senior author on the study. From his lab, co-authors included lead author Fang Yang and N. Adrian Leu. The Penn researchers teamed with Sherman Silber from St. Luke’s Hospital in St. Louis, Robert D. Oates from Boston University Medical Center and Janet D. Marszalek, Helen Skaletsky, Laura G. Brown, Steve Rozen and David Page from Whitehead Institute at the Massachusetts Institute of Technology.
The paper is published in the journal EMBO Molecular Medicine. P. Jeremy Wang, a professor in the Department of Biomedical Sciences at Penn’s School of Veterinary Medicine, was the senior author on the study. From his lab, co-authors included lead author Fang Yang and N. Adrian Leu. The Penn researchers teamed with Sherman Silber from St. Luke’s Hospital in St. Louis, Robert D. Oates from Boston University Medical Center and Janet D. Marszalek, Helen Skaletsky, Laura G. Brown, Steve Rozen and David Page from Whitehead Institute at the Massachusetts Institute of Technology.
The study has its roots 15 years ago, when Wang and colleagues cloned the Tex11 gene and found that it was specific to germ cells and was located on the X chromosome. In 2008, his group published a study showing that disrupting Tex11 function caused sterility in male mice and caused female mice to have smaller litters. This disruption halts the maturation of germ cells by interfering with meiosis, the process by which an individual’s genetic material is divided and sorted into what eventually becomes eggs and sperm.
To further extend these findings’ implications for humans, Wang’s team screened genomic samples from 246 men with azoospermia as well as others to serve as controls, looking for variations in the TEX11 gene. They found more variants in men with azoospermia than in the controls, hinting that the protein plays a key role in sperm development in humans.
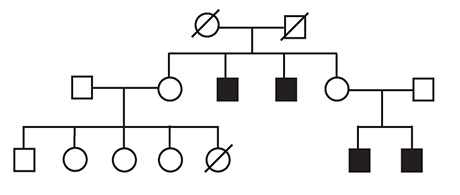
Though it can be difficult to create pedigrees of infertility for the very reason that men with the condition cannot have children, the researchers did find a person carrying a mutation in the TEX11 gene who had a compelling family history. Both he and his brother were azoospermic, and a genetic sample from his mother showed that she was heterozygous for the TEX11 mutation. Both of his maternal uncles were childless, though the researchers didn’t have their genetic material to confirm they had the same mutation. But the pattern is indicative of a trait passed down the maternal line on the X chromosome, inherited from the mother.
“Because these two maternal uncles are sterile, ultimately, we believe that it traces back to the individual’s grandmother,” Wang said.
To experimentally test whether the mutations that were identified in infertile men were the reason for their azoospermia, the research team selected three mutations found in the azoospermic men but not in the controls. They then engineered mice with versions of Tex11 that bear those same mutations and experimentally bred mice to express those mutated versions.
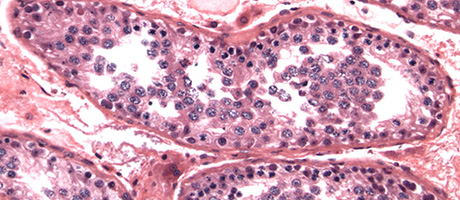
For two of these mutations, they saw little effect. But the third had striking effects: males with this variant had significantly lower sperm counts than control animals. In addition, these mice had abnormalities in meiosis.
In total, from the screening of 275 men, researchers found three mutations in TEX11 that appeared to be the cause of the infertility, resulting in a rate of about 1 percent of cases of azoospermia.
“Given that there are hundreds of candidate genes for male infertility,” Wang said, “1 percent is actually very significant.”
As a trend toward personalized medicine means that more people can have portions or the entirety of their genome sequenced, these results have implications for genetic counseling for infertility.
“If men had one of these same mutations in this gene, I think we could safely say that’s the cause of their infertility,” Wang said.
The study was supported by the Howard Hughes Medical Institute and National Institutes of Health’s National Institute of General Medical Sciences.