 |
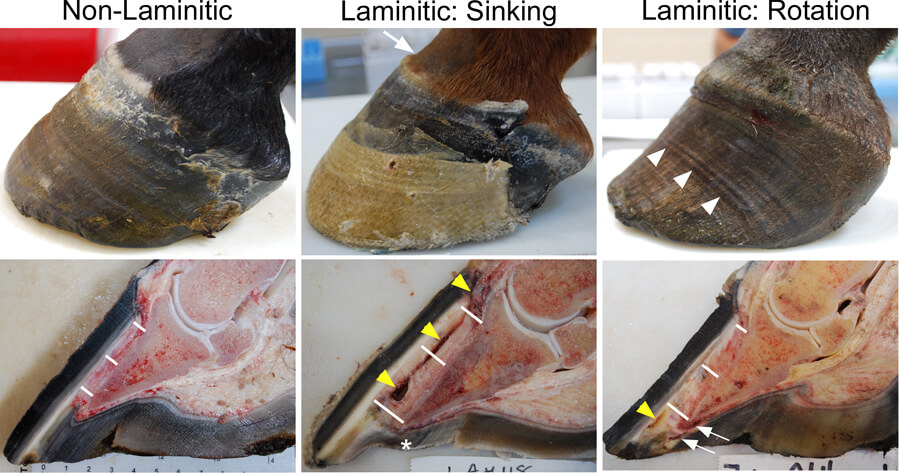
Laminitis Gross Figure Left front feet from a non-laminitic 2 year old Thoroughbred gelding (Left), a 2 year old Thoroughbred filly with acute supporting limb laminitis (Middle), and a 14 year old Warmblood/Thoroughbred-cross mare with chronic endocrinopathic laminitis (Right). The non-laminitic foot has a normal exterior appearance and the distance, denoted by the white bars, from the base of the lamellae/inner limit of the hoof wall to the surface of the coffin/distal phalanx bone is approximately 5 mm at proximal, middle, and distal locations along the hoof wall. The foot from the filly with supporting limb laminitis shows evidence of sinking of the distal phalanx within the hoof capsule: “clefting” (arrow) at the coronary band, detached lamellae leaving a gap and hemorrhage (yellow arrowheads) between the hoof (epidermal lamellae) and the dermal tissue, increased distance all along the base of the lamellae to the distal phalanx (proximal: 10 mm, middle: 12 mm, distal: 15 mm), and a dropped sole (*). The foot from the mare with chronic endocrinopathic laminitis shows evidence of rotation of the distal phalanx within the hoof capsule and repeated episodes of acute laminitis, as evidenced by multiple “founder rings” (white arrowheads), increasing distance from the base of the lamellae to the distal phalanx, such that the distal phalanx has rotated away from the hoof capsule (proximal: 6 mm, middle: 8 mm, distal: 10 mm), serum in the hoof white line from detaching lamellae distally (yellow arrowhead), and crushed dermal tissue and solar abscess formation under the toe of the distal phalanx (white arrows). All images are from naturally-occurring cases in the Laminitis Discovery Database, which has been supported by many horse owners and referring veterinarians who hoped that we could learn something from these tragic cases, and from our financial support from donors to the Laminitis Research Fund at Penn Vet, Grayson-Jockey Club Research Foundation, and the Bernice Barbour Foundation. |
|
|
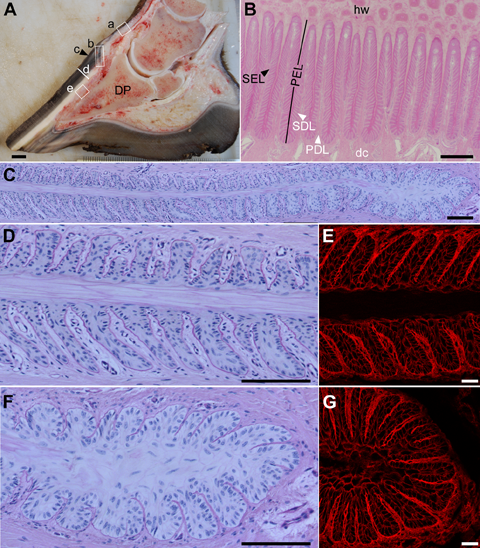
Gross and microscopic anatomy of the non-laminitic equine foot and the hoof lamellae
A) Midsagittal section of a non-laminitic equine foot indicates the anatomical locations of tissue samples retrieved for the LDD (boxed) and associated anatomical structures: a) haired skin of the pastern, b) coronet, c) periople, also called the stratum externum layer of the hoof capsule, d) hoof wall, also called the stratum medium layer of the hoof capsule, e) lamellar tissue sample, including 1-2 mm of hoof wall (hw), epidermal lamellae, also called the stratum internum layer of the hoof capsule, dermal lamellae, and dermal corium up to the surface of the distal phalanx (DP).
B) Histological transverse section of the lamellar region, stained with hematoxylin and eosin. Hoof wall (hw) is at the top of the image and is continuous with the cornifying primary epidermal lamellae (PEL). Each PEL has approximately 150 secondary epidermal lamellae (SEL; black arrowhead). PELs and SELs increase the surface area of attachment between the hoof capsule and connective tissue which suspends the DP. The PELs and SELs interdigitate with primary dermal lamellae (PDLs) and secondary dermal lamellae (SDLs) which are in turn continuous with the dermal corium (dc).
C-G) Histological transverse section of the middle and axial (adjacent to the DP) regions of a single PEL with its associated SELs, SDLs, and adjacent PDLs, stained with periodic acid-Schiff and hematoxylin. D, F) Higher magnification of D) the middle (left side of (C)) region of a PEL and E) the axial (right side of (C)) region of a PEL composed of the central cornifying keratinized axis (PEL KA), Hbranching SELs and interdigitating SDLs with an intervening basement membrane zone (BM), and adjacent PDL. E, G) Similar regions are imaged by confocal microscopy using a rhodamine-conjugated wheat germ agglutinin counterstain developed in the laminitis laboratory. The counterstain allows the visualization of PDL and SDL connective tissue and the perimembranous regions of SEL cells.
Scale bars: A: 1 cm; B: 500 μm; C, D, E: 100 μm.
|
|
|
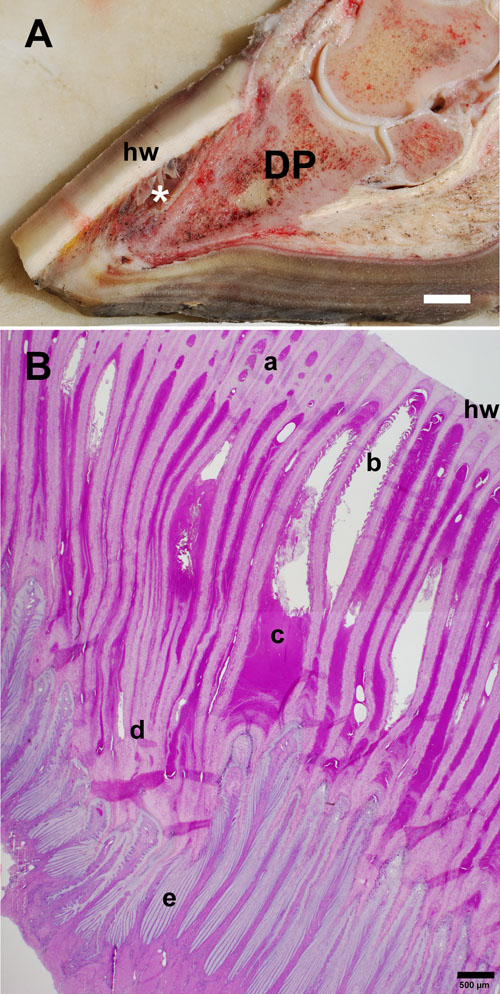
Gross and microscopic anatomy of the laminitic equine foot and hoof lamellae from a mare with suspected equine metabolic syndrome and endocrinopathic laminitis of 8 weeks’ duration
A) Laminitis results in failure of the suspensory apparatus of the distal phalanx (DP) and can lead to ventral rotation and/or sinking of the distal phalanx relative to the dorsal hoof wall (hw). The lamellae are forming a hyperplastic/metaplastic lamellar wedge of cornified tissue (*) in response to epidermal lamellar tissue damage. Weight bearing is largely shifted from the hoof wall to the sole, causing tissue damage and pain. (Scale bar = 1 cm.)
B) Histological transverse section from the mid-dorsal lamellar region, stained with periodic acid-Schiff/hematoxylin. The cut edge of the hoof wall is to the upper right. New cap horn tubules (a) form between the primary epidermal lamellae (PEL) adjacent to the hoof wall and partially fill the defect (b) created by dermal-epidermal and epidermal cell-cell detachment. Detached, necrotic SELs (b) and serous exudate (c) fill large tissue clefts, providing an ideal environment for bacterial colonization and abscess formation. The extremely elongated PELs (d) and SELs (e) give rise to the dysplastic, hyperplastic, and hyperkeratotic (excessively cornified) epidermal tissue of the lamellar wedge.
(Scale bar = 500 µm.)
|
|
|
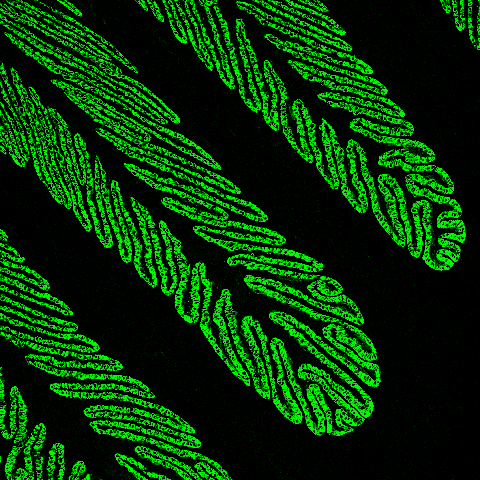
The horse’s hoof lamellae
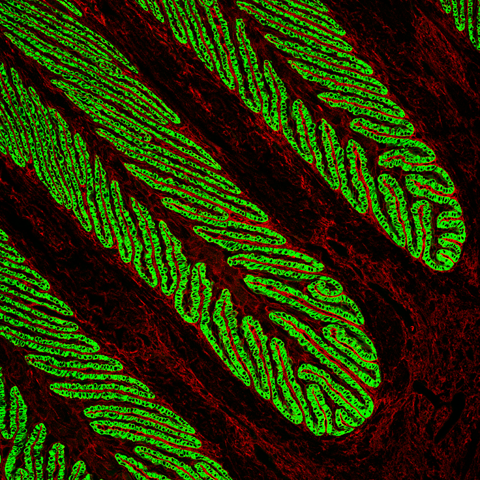
The horse’s hoof lamellae consist of primary and secondary folds (PELs and SELs), resembling a pine tree. This unique evolutionary adaptation increases the contact area between the hoof and underlying tissues, allowing the transfer of the horse’s weight from the bones of the limb to the hoof. As horses evolved from animals with five toes to much larger animals with just one toe, this increased folding of the inner hoof was necessary to maintain hoof attachment. In this confocal image, the basal cells of the SELs are stained green using an antibody against an epithelial basal cell keratin, K14. We have shown that K14 is also expressed in equine skin, but its expression is not so tightly coupled to the basal cell layer. We believe that this keratin expression reflects the much more abrupt differentiation of lamellar cells to terminally differentiated cornifying cells in normal lamellae, a differentiation phenotype that is disturbed by laminitis. In both skin and laminitic lamellae, K14 expression extends into the suprabasal cell layers, consistent with altered differentiation.
|
|
| Because the epidermal and dermal lamellae are interdigitating and mirror each other, it can be difficult to orient to cell types in lamellar tissue. We developed a rhodamine-conjugated wheat germ agglutinin (WGA) counterstain that facilitates visualization of the dermal connective tissue of the PDLs and SDLs. When compared to the previous image, the red WGA dermal connective tissue counterstain in this image indicates that the image depicts the PEL tips closest to the DP rather than the bases of the PELs at the hoof wall. |
|
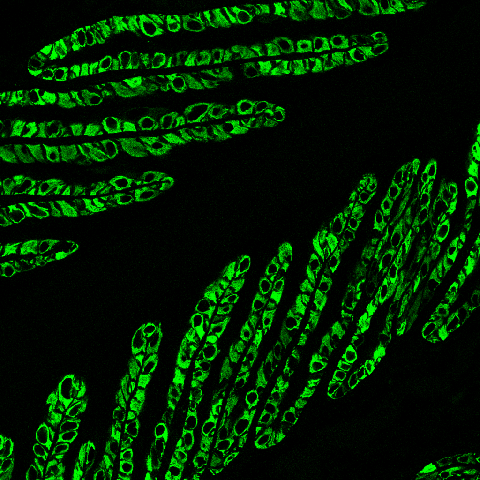
This confocal image shows the localization of the basal cell keratin, K14 (green) to the cytoplasm of the SEL basal cells. The black space in the center of the figure is the keratinized axis of the PEL. Keratins are the major cytoskeletal proteins of the SELs and the major source of mechanical strength in these and other epidermal cells such as skin cells. In addition to the basal keratins, a proteomic analysis of lamellar tissue identified a novel keratin pair, K124 and K42, as the major cytoskeletal proteins of SELs, accounting for more than 50% of keratin content. PCR studies in the lab have shown that these keratins are uniquely expressed in the lamellae, are not expressed in skin, and could therefore serve as tissue-specific biomarkers for laminitis.
|
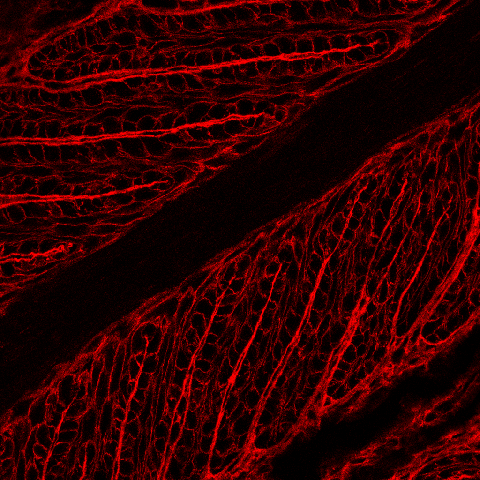 |
| This is the same lamellar tissue as shown in the previous image demonstrating localization of rhodamine-conjugated WGA to the dermal connective tissue of the SDLs and PDLs as well as to the perimembranous regions outlining the SEL cells. This counterstain helps us determine the exact subcellular localization of proteins that localize to SEL cells, as shown in the next image. |
.png?sfvrsn=f2df1aba_2) |
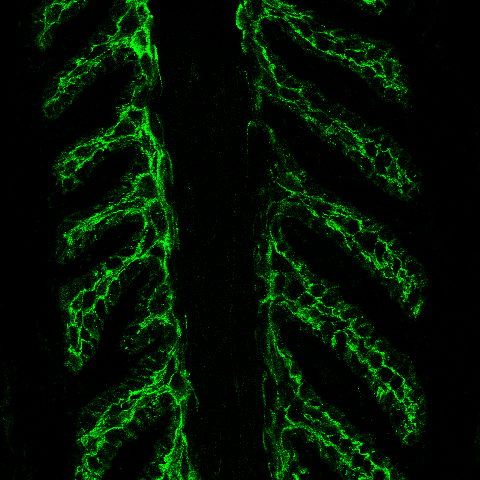
| Merging the previous two confocal images allows us to precisely localize the SDLs, SELs, and to see that the basal keratin, K14, localizes very precisely to the SEL basal cell cytoplasm where it, along with other keratins, forms the major cytoskeletal mechanical support of the lamellae. K14 staining does not extend to the nucleus (black oval/circular region) or to the basal cell membranes (stained red with WGA counterstain – can be seen outlining epidermal cells). |
 |
| In addition to the specialized keratin cytoskeletal proteins of the SELs, we are interested in the effect of laminitis on epidermal cell-cell adhesion. SEL cells, like the skin epidermis, are anchored to each other by intercellular adhesion complexes called desmosomes. In this image, the green stain shows the location of one of the desmosomal proteins, desmoplakin, in normal lamellar tissue. Desmoplakin anchors the keratin cytoskeleton to the desmosomal complex and therefore localizes to the perimembranous region of SEL cells. We are investigating the effect of laminitis on the expression and localization of desmoplakin. |
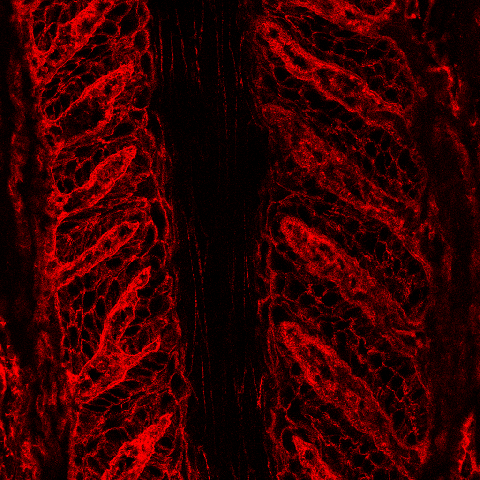 |
| As with the previous series of images, applying our rhodamine-conjugated WGA counterstain allows us to more precisely localize PDL and SDL connective tissue and the perimembranous regions of SEL cells. |
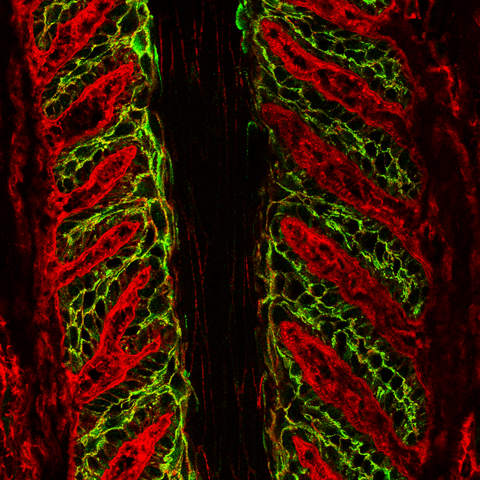 |
| Merging the previous two images allows us to show that desmoplakin (green) localizes to the perimembranous regions of SEL cells (red). Overlapping staining of red (WGA) and green (desmoplakin) appears yellow. |
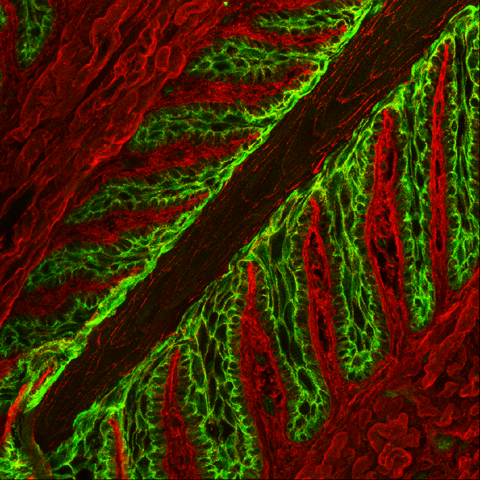 |
| While the previous images represent single “slices” from confocal imaging (i.e., two-dimensional, X and Y axes images), this image is a z-stack of several slices to generate a three-dimensional, X, Y and Z axes images. As for the previous images, the green stain identifies desmoplakin localization at the periphery of SEL cells, anchoring the keratin cytoskeleton to desmosomal adhesion complexes, and the red stain is our WGA counterstain, showing the 3D structure of the dermal connective tissue and SEL perimembranous regions. |