The mosquito has multiple lines of defense against invading pathogens. One potent barrier is found in its blood, called hemolymph (Figure 1).
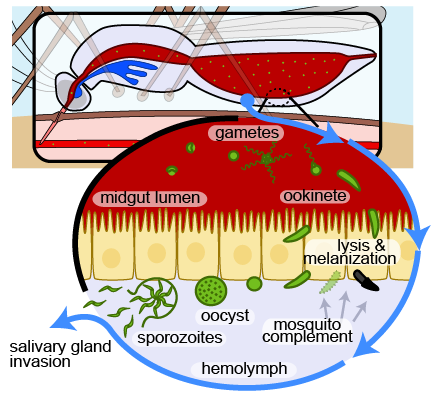
Parasites migrate through the gut epithelium in order to escape the harsh digestive conditions of the gut lumen. Here they come into contact with the hemolymph. At this point parasites are recognized and killed by lysis or melanization. Those that survive transform into oocysts. Within the cyst wall parasites replicate. Approximately two weeks later, oocysts burst each releasing thousands of sporozoites into the hemolymph where they migrate to the salivary gland to injected into the next when the mosquito takes its next blood meal.
The hemolymph barrier is formed by a network of proteins referred to as the complement-like pathway due to its similarities to the vertebrate complement pathway. Two leucine-rich repeat (LRR) containing proteins, LRIM1 and APL1C, are essential are essential components of the mosquito complement pathway. These proteins circulate in the mosquito hemolymph in a disulfide-bonded dimer (Figure 2).
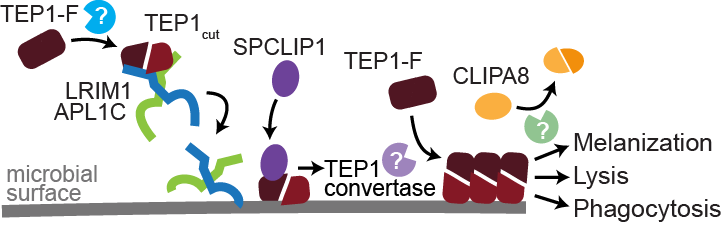
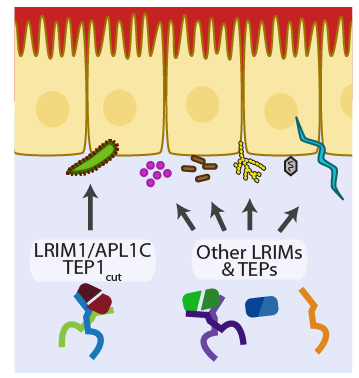
If either LRIM1 or APL1C is silenced by RNAi, the complex is undetectable in the hemolymph and parasite survival is massively increased. Before parasites are killed, the complement C3-like protein TEP1 is localized on their surface, marking them for destruction. The LRIM1/APL1C complex physically interacts with a proteolytically processed and highly reactive form of TEP1 called TEP1cut. The enzyme(s) responsible for the generation of steady-state levels of TEP1cut are not known. The interaction stabilizes TEP1cut in the hemolymph and is required for its localization to parasites during midgut invasion. When the LRIM1/APL1C complex is knocked-down, TEP1 fails to localize and the invading parasites are not killed. This immune pathway leading to parasite killing could be an important cause of natural refractoriness in non-vector mosquitoes. We recently discovered that the LRIM1/APL1C complex can also interact with 3 other members of the TEP family. Two of these were previously characterized to contribute to mosquito antibacterial defense reactions. We found that one of these, TEP3, also functions in mosquito immune reactions against parasites.
Additionally, we have found that LRIM1 and APL1C are defining members of a protein family, collectively named LRIMs (pronounced L-rims). Bioinformatic searches using specific features shared between LRIM1 and APL1C has uncovered approximately 20 family members falling into four distinct sub-families in the mosquito species Anopheles gambiae, Aedes aegypti and Culex quinquefasciatus (Figure 3).
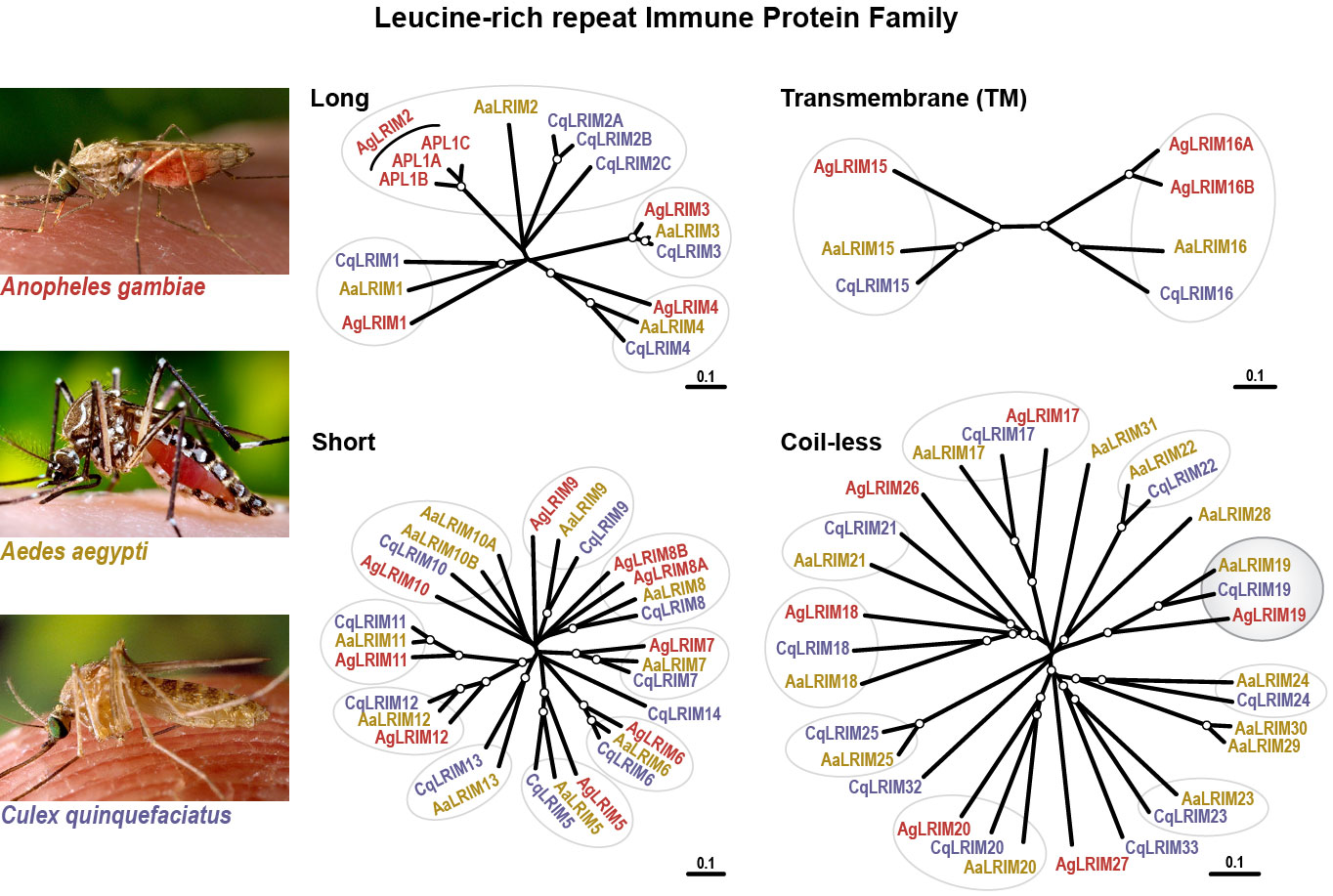
This family is not found in any other organism. Given the central role of LRR proteins in host defense in plants and animals, we are currently investigating the hypothesis that the repertoire of LRIMs may help the mosquito neutralize diverse pathogens, including the agents of human and animal diseases that they transmit (Figure 4).
We have recently discovered a novel branch of the immune system that is specifically regulated by the steroid hormone 20-hydroxyecdysone (20E). 20E is produced transiently in adult female mosquitoes in response to blood feeding and has a well-characterized role in egg production (Figure 1)
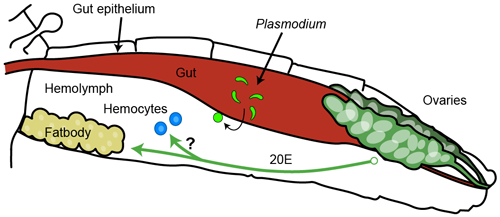
We found that the 20E also regulates the immune protein LRIM9. LRIM9 protein is strongly induced in the mosquito hemolymph after blood feeding and protects the mosquito from infection by malaria parasites. Preliminary analysis revealed that LRIM9 is not part of the mosquito complement-like pathway and the mechanism by which is restricts parasite development is not known.
We are currently investigating
- the mechanism of how LRIM9 leads to lower infection by Plasmodium
- whether 20E regulates immune pathways in the mosquito hemolymph or other tissues
Mosquitoes are essential for the canine heartworm,
Dirofilaria immitis,
life cycle serving as both an intermediate hosts and vectors. Transmission requires that ingested
D. immitis microfilariae develop into infective third-stage larvae (L3) over an approximately 2-week period. As it develops in the mosquito,
D. immitis interacts with different tissues and immunological barriers. We are addressing the role of immune signaling pathways in controlling
D. immitis infection in the model vector
Aedes aegypti.
Current Lab Members
- Dr. Sutopa Dwivedi, Postdoctoral researcher
- Elizabeth Edgerton, Lab technician
- Sarah Sneed, PhD student, IGG program
- Greg Sousa, VMD/PhD student, MVP program
- John Filosa, CAS ’19, Undergraduate researcher, College House Research Fellow
- Barbara Biney, CAS ’18, Undergraduate researcher
- John Cain, VMD student
- Sophia Reeder (rotation), PhD student, IGG program
Former Lab Members
Letitia Thompson, Lab technician 2014-16
Currently in graduate school at NYU. Letitia managed the lab for two years and was in charge rearing our mosquitoes. In that time, I estimate Letitia produced approximately one million mosquitoes! Letitia also characterized complement proteins in Aedes aegypti.
Ariel Aguiar, PennVet ’18, Merial Summer Scholar 2016
Ariel studied whether the mosquito’s immune contributes to the refractory phenotype of Aedes aegypti Liverpool strain to infection by canine heartworm infection
Antonia Bass, MVP program, Rotation student Fall 2015
Antonia investigated complement regulation in Anopheles gambiae
Kristin Privette (Derfus), volunteer Summer 2015
Kristin is investigating the genetics of susceptibility of Aedes aegypti mosquitoes to infection by canine heartworm Dirofilaria immitis
Andrew Hart, SUIP student, Summer 2015
Andrew studied the complement pathway in male Anopheles gambiae. Andrew gained early acceptance to Penn IGG PhD program.
Jenny Kwok, PennVet ’17, Merial Summer Scholar 2015
Jenny studied how activation of the mosquito's immune signaling in the affects infection by canine heartworm Dirofilaria immitis in Aedes aegypti. Jenny won first prize for her Research Day presentation.
Valeria Reyes Ruiz, Graduate student, MVP program, JoinedSunny Shin's laboratory.
Valeria studied the specificity of mosquito complement activation (Rotation student, Spring 2015)
Collaborators
Megan Povelones, Penn State University, Brandywine