Cancers are characterized by uncontrolled growth of cells, but such expansive growth is also a necessary feature of repair and regeneration. Dr. Christopher Lengner, Associate Professor in Penn Vet’s Department of Biomedical Sciences, has spent the past decade exploring how stem cells play a role in both biological processes.
 “My lab has worked on studying the intestinal epithelium in colorectal cancer on the one hand and regeneration after injury on the other,” Lengner said. “These are two sides of the same coin, with the notion that the stem cell is the underlying thread that ties the two together.”
“My lab has worked on studying the intestinal epithelium in colorectal cancer on the one hand and regeneration after injury on the other,” Lengner said. “These are two sides of the same coin, with the notion that the stem cell is the underlying thread that ties the two together.”
Colorectal cancer is the second leading cause of cancer-related death in the United States; more than 130,000 new cases are expected to be diagnosed this year. As a member of Penn Vet’s new Cancer Center, Lengner is working to illuminate the driving forces of this disease, with an aim of developing new targets for therapies that kill cancer cells while sparing the healthy functions of the dynamic intestinal tissues. Using cutting-edge technologies and collaborating with human clinicians within Penn’s Perelman School of Medicine, he’s translating findings in mice to identify treatment strategies that may one day help people.
Lengner began his career investigating the mechanisms that govern the ability of stem cells to differentiate into a variety of cell types—and the factors that can go awry with this process in disease states. That work led him to a focus on how cancer gets its start.
“There is fairly good evidence now that when an oncogene gets mutated it only develops into cancer if it gets mutated in astem cell. And when a tissue is injured, it can only regenerate through the activity of a stem cell,” Lengner explained. “Therefore, the beneficial effects of being able to regenerate a tissue after injury come at the cost of being
susceptible to cancer.”
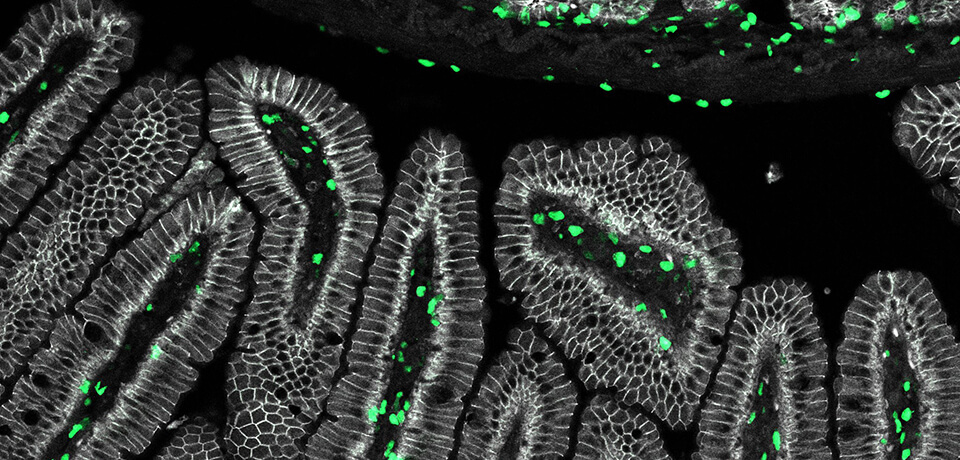
Lengner’s recent studies have illuminated the dual nature of stem cells. Along with colleagues, he has examined the RNA-binding proteins MSI1 and MSI2, part of the Musashi family, finding them to be crucial drivers of colon cancer. Blocking their activity, the researchers found, makes mice resistant to cancer. But the story isn’t so simple. While mice with both genes deleted initially appeared healthy, the animals were unable to recover from radiation injury, as their ability to regenerate intestinal tissue was severely compromised.
The work underscored the importance of so-called “reserve stem cells,” which represent a tiny fraction of intestinal epithelial cells but have an outsize role in regeneration. These cells are believed to be long-lived and resistant to radiation and chemotherapy due to being in a state of dormancy, with their genetic material tightly packed and thus protected. Deleting the Musashi genes in only the reserve stem cells deprived mice of the ability to regenerate intestinal epithelial tissue after injury. Further study showed that the MSI genes were required for reserve stem cells to leave dormancy and enter the cell cycle.
The findings point to a strategy whereby intestinal tissues could be shielded from damage prior to radiation by keeping them dormant, thus promoting tissue regeneration once the treatment is completed. But they also hint at a way in which a theoretical cancerous reserve stem cell might “hide out” in the tissues in a quiescent state and avoid being killed by radiation or chemotherapy.
Long-lived cells like the reserve stem cells are vulnerable; the longer they live, the higher the chance they will be exposed to cancer-inducing mutagens. This process can take years—even decades—to occur in people. That makes it hard to mimic in mice.
“A lot of people have said mice don’t make good models of human cancer,” Lengner noted. “And the more I thought about it, the more I think the reason is because they’re doing it wrong.”
In most cases, scientists have induced cancer in mice by introducing an oncogenic mutation in thousands of cells at once. Lengner’s team is instead attempting to model human disease in mice in a more naturalistic fashion, mutating a single cell and monitoring it as it divides. They’re also contemplating other factors that play into tumorigenesis in people—for example, environmental stressors such as inflammation and nutrient excess.
Early results from Lengner’s lab suggest that an inflammatory environment may coax a normally non-disease-causing mutation to initiate a process that leads to tumor growth. In addition, building on findings that suggest that fasting or calorie restriction improve the results of chemotherapy, they are examining how nutrient levels could tip the balance of dormant to active stem cells. While active cells may be more susceptible to cancer treatment, a dormant cancerous cell may be able to “hide” from therapies, forming the basis for cancer regrowth even after an apparently successful treatment.
To uncover the mechanisms of oncogenesis, Lengner has embraced the use of new techniques and tools to efficiently manipulate and evaluate cells and tissues. In particular, the CRISPR-Cas9 system—a technique that allows researchers to edit the genome by adding or removing specific portions of DNA—and organoids—a growth in vitro that resembles an organ—are helping Lengner and his colleagues advance cancer work.
“By combining these technologies, we now have a genetically controlled system where you can test therapies, antibodies, and additional genetic factors that may or may not drive or prevent metastatic disease,” he said.
Through a collaboration with Dr. Anil Rustgi, Division Chief of Gastroenterology, and others at Penn Medicine, Lengner plans to use both normal and cancerous organoids to evaluate the effectiveness of various compounds as possible cancer therapies.
Addressing cancer, he emphasized, requires a team approach. “Really successful things happen when you have people focused on different areas, bringing their respective expertise together.”