 William Beltran, Artur Cideciyan, Gustavo Aguirre, and Samuel Jacobson were part of the joint team from Penn Vet and Penn Medicine
William Beltran, Artur Cideciyan, Gustavo Aguirre, and Samuel Jacobson were part of the joint team from Penn Vet and Penn Medicine
who led the work.
The last year has seen milestones in the gene therapy field, with FDA approvals to treat cancer and an inherited blinding disorder. New findings from a team led by University of Pennsylvania vision scientists, who have taken gene therapies into clinical trials in the past, are proving successful, this time treating a form of retinitis pigmentosa, a disease that progressively robs people of their night and peripheral vision before blindness develops.
The researchers, from Penn’s School of Veterinary Medicine and Perelman School of Medicine, in collaboration with University of Florida scientists, developed a therapy that effectively eliminates the abnormal copy of rhodopsin, a light-sensing molecule, and then restores it with a healthy copy of the protein. This knockdown and replacement approach preserved the retina’s light-sensing photoreceptor cells in affected dogs, which can develop a very similar disease to affected humans.
What’s more, they accomplished this using a single viral vector to co-deliver the genetic material needed to achieve both the knockdown and replacement. Though more than 150 different mutations in rhodopsin have been identified to cause retinitis pigmentosa, this approach is intended to work regardless of the mutation or the mechanism by which rod photoreceptor cells, those responsible for vision in dim light, die. That means that a large percentage of patients with rhodopsin autosomal dominant retinitis pigmentosa could benefit if the therapy is found to be safe and effective in people.
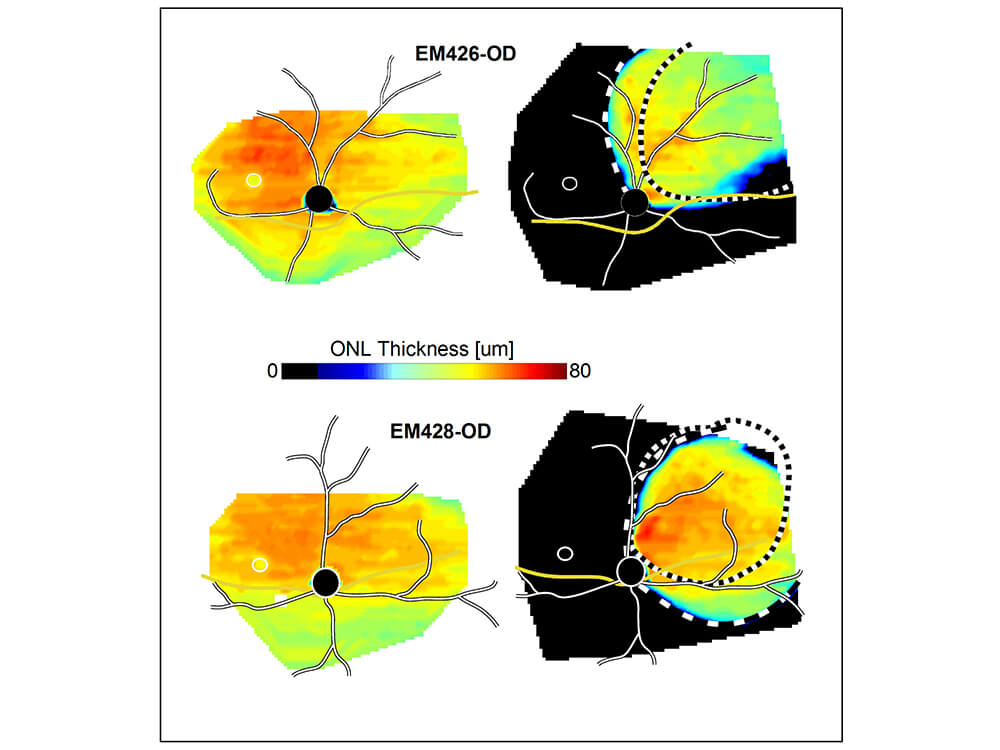 Maps reflecting the thickness of a key layer of the retina show how a gene therapy treatment (right panels) protected against
Maps reflecting the thickness of a key layer of the retina show how a gene therapy treatment (right panels) protected against
severe retinal degeneration.
“It’s a ‘one treatment fits all,’” says William A. Beltran, professor of ophthalmology and director of the Division of Experimental Retinal Therapies at Penn Vet, and co-lead author of the study, which appears in this week’s Proceedings of the National Academy of Sciences. “The treatment targets a region of the rhodopsin gene that is homologous in humans and dogs, and is separate from where the mutations are located. That gives us great hope about making this a translational treatment.”
“We’ve known for decades that this specific molecule causes a specific form of retinitis pigmentosa, but developing a treatment has not been straightforward,” says Artur V. Cideciyan, research professor of ophthalmology at Penn Medicine and co-lead author. “Now, with these elegant results based on years of study in dogs, we can start working toward treating these mutations and prevent deterioration of photoreceptor cells in humans.”
 Raghavi Sudharsan
Raghavi Sudharsan
The work is the latest result in a long-standing partnership among Penn vision scientists. In addition to Beltran and Cideciyan, the team included Penn Vet’s Raghavi Sudharsan, a research associate in the Beltran lab, and Gustavo D. Aguirre, professor of medical genetics and ophthalmology, and Penn Medicine’s Samuel G. Jacobson, professor of ophthalmology and director of the Center for Hereditary Retinal Degenerations.
Retinitis pigmentosa refers to a set of progressive hereditary retinal disorders. Three decades ago, researchers identified mutations in the gene encoding rhodopsin as the first known genetic cause of the condition. Other genes have since been implicated as well, but rhodopsin mutations remain a major contributor, accounting for up to 30 percent of autosomal dominant retinitis pigmentosa.
The vast majority of rhodopsin mutations are passed through families in a dominant fashion, meaning that a parent need only pass one mutated copy on for their child to be affected.
“In our investigations, we’ve seen people in the 1990s with this genetic type of retinitis pigmentosa and now we’re seeing their grandchildren also affected,” Jacobson says. “It’s a multi-generational disease, and it’s a serious disease.”
Several rhodopsin mutations that lead to retinitis pigmentosa result in what’s known as a toxic gain-of-function, thought to produce a protein that is harmful to the photoreceptor cells. In order to address the problems that arise in these patients, researchers have determined that the best strategy is to eliminate the mutant protein.
“We’ve developed in the past gene therapies for other conditions where the mutation causes a loss of function,” says Aguirre, “so in these cases, we just needed to add back the normal copy of the gene in order for the photoreceptors to regain their normal structure and function. When you have a dominant disease like this one, where the gene product is really damaging to the cell, you have to get rid of it.”
Earlier efforts by the team had attempted to do just that: simply knockdown both the mutant and normal rhodopsin in dogs. This prevented the disease but caused a loss of the key cellular compartment of rod photoreceptors, the outer segment, which initiates vision. Beltran and colleagues determined that adding back a healthy copy would be the best and necessary strategy to achieve healthy functioning retinal cells.
In the current work, the researchers employed this strategy, which relied on both in vitro assays and, significantly, a canine animal model with a naturally occurring mutation in the rhodopsin gene that faithfully recapitulates the form of human retinitis pigmentosa caused by rhodopsin class B mutations. While patients with class A mutations in rhodopsin lose functioning rod photoreceptor cells early in life, those with class B mutations may retain their rod cells for decades, making them candidates for a vision-preserving gene therapy.
Because mutant rhodopsin dogs are very sensitive to ambient light, a short exposure equivalent to midday luminance was used to accelerate the degeneration of photoreceptors. This sensitivity allowed the researchers to control which areas of the retina were most affected and when, and thus evaluate treatment success in a matter of weeks rather than the years it would take to develop degeneration without this trigger.
In the lab, the team confirmed that they could disrupt the mutant rhodopsin gene using a knockdown reagent called short-hairpin RNA (shRNA), engineered by Alfred Lewin and colleagues at the University of Florida, to target an area away from the mutated sections of the gene. Adding back a healthy copy of the rhodopsin gene that is resistant to this shRNA compensated for the knockdown.
In dogs with the rhodopsin mutation, they used the same strategy, finding the best success when both the shRNA and the healthy copy of rhodopsin were co-delivered in the same vector, as opposed to using two different vectors. The team restored roughly 30 percent of the normal level of rhodopsin, enough to prevent deterioration of rod cells in the retina.
“What we showed was that if you just did the knockdown alone, you preserve the outer nuclear layer of rods, which is where the cell bodies are located,” Beltran says. “But without another critical layer, the outer segments, where rhodopsin plays the essential role of capturing light and initiating vision, then the rods become useless. However, if you combine the knockdown with the replacement reagents, then the drastic difference is that you now have perfectly formed and aligned outer segments and functional photoreceptor cells.”
The researchers were able to confirm the beneficial effects on both structure and function of rod cells (as well as cones, responsible for color vision) by using specialized imaging techniques that can be used in human patients, along with electroretinography, which provides a measure of rod and cone function.
“We were able to save cone vision in the dogs,” says Florida’s Lewin. “If we can do that in people, it will save the central vision that allows them to recognize faces, read and watch television.”
Thus far, tracking the treatment effect more than eight months after delivery of the gene therapy, the effect seems stable and lasting. The research team is currently working to move the findings into clinical trials.
“The current work has strong implications for the treatment of patients with autosomal dominant retinitis pigmentosa due to Class B rhodopsin mutations,” Cideciyan says.
Additional authors on the study were Penn Vet’s Valérie Dufour, Simone Iwabe, Luis Felipe Marinho, and Tatyana Appelbaum; Penn Medicine’s Malgorzata Swider, Brianna Lisi, and Alexander Sumaroka; and University of Florida’s Michael Massengill, and Brian Rossmiller.
The research was supported by the National Eye Institute (grants EY022012, EY06855, EY001583, and EY021721), Foundation Fighting Blindness, Research to Prevent Blindness, and the Shaler Richardson Professorship Endowment.