Andrew van Eps, BVSc, PhD, graduated from the University of Queensland School of Veterinary Science in Australia. He remained there to carry out his PhD studies, which primarily focused on the effects of therapeutic hypothermia on the development of laminitis. After completing a large animal medicine residency at New Bolton Center, he returned to the University of Queensland as a faculty member for seven years, before returning to New Bolton Center (NBC) in 2017 as an associate professor of musculoskeletal research in the Department of Clinical Studies (NBC). Dr. van Eps’ research goal is to identify the key pathophysiological events that lead to different forms of laminitis in order to develop clinically applicable means of preventing this crippling equine disease.
Laminitis: an important equine foot condition with diverse clinical causes
Laminitis is a major cause of morbidity and mortality in horses worldwide. In a normal hoof, connecting tissues called lamellae attach to the coffin bone, suspending it within the hoof capsule. In laminitis, failure of the lamellar attachment leads to a largely unrecoverable loss of this suspensory function, resulting in chronic and often progressive lameness and dysfunction that may ultimately require euthanasia. Laminitis occurs secondary to a diverse array of primary disease states, which primarily fit into 3 main categories: 1) sepsis-related laminitis (SRL); 2) endocrinopathic laminitis (associated with insulin dysregulation/hyperinsulinemia) and 3) supporting limb laminitis (SLL). Over the last decade, we have recognized key differences (and some similarities) in the initial events that lead to these three types of laminitis. A focus on these early events is leading to a better understanding of why laminitis occurs in different clinical situations and is helping to identify therapeutic targets.
What causes lamellar attachment failure in different forms of laminitis?
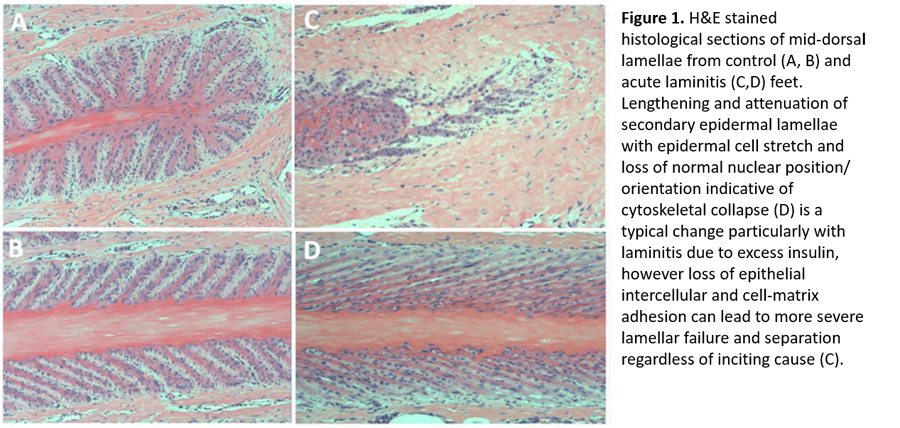
In each form of laminitis, a combination of epithelial cell adhesion loss and epithelial cell stretch weakens the lamellar attachment (Figure 1). Endocrinopathic laminitis is dominated by cell stretch, whereas SRL and SLL are dominated by adhesion loss. A question of vital importance to our understanding is whether this attachment failure occurs as a result of 1) a specific cellular signaling event that is common to all forms of laminitis; or 2) insult-specific alterations in epithelial cell homeostasis that lead to a common endpoint due to the unique and immense physical stresses on the lamellar epithelium. The van Eps laboratory is taking a multidisciplinary approach to examining structural, molecular and physiologic events during laminitis development to answer this fundamental question.
Cellular energy failure a likely candidate but is it a consistent feature?
The maintenance of lamellar epithelial adhesion and cytoskeletal dynamics required to withstand the immense mechanical stress within the equine hoof is an energy consuming process. Using our recently developed tissue microdialysis system capable of assessing lamellar perfusion and energy balance in vivo, we have demonstrated a relatively high rate of glucose consumption and lactate production within the lamellae[1]. As lamellar epithelial cells are highly dependent on glucose as an energy substrate[2] and there is no means for local storage of glycogen in the lamellar tissue, lamellar epidermal glucose uptake must be matched by constant delivery via the blood to maintain function. However, our microdialysis urea clearance data demonstrate that even the normal lamellar dermis is relatively poorly perfused compared with the adjacent sublamellar dermis or skin [1], making it inherently prone to energy imbalances secondary to perfusion or metabolic derangement. The van Eps lab has therefore focused on determining whether this contributes to the development of different forms of laminitis.
Evidence for ischemia only in supporting-limb laminitis
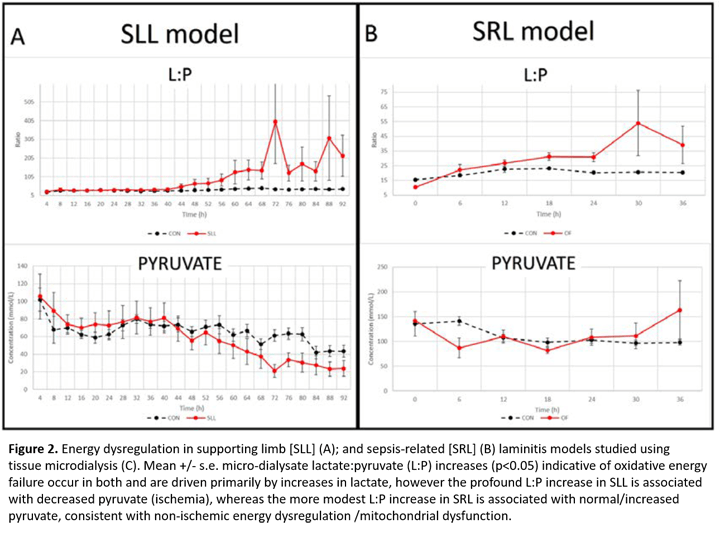
While perfusion derangements have long been implicated in different forms of laminitis, direct evidence has been lacking. Using the lamellar tissue microdialysis technique described above, we have demonstrated a unique relationship between limb load cycling and lamellar perfusion [3]. Our new data reveal lamellar ischemia in a preferential weight bearing model relevant to SLL, characterized by an increased lamellar interstitial lactate:pyruvate (L:P) ratio coupled with reduced urea clearance (Figure 2A) providing the first evidence of ischemia/perfusion failure in the development of SLL. Notably, our microdialysis studies suggest ischemia is not a primary event in the development of SRL (oligofructose (OF) model) [4] or endocrinopathic laminitis (hyperinsulinemic clamp model, unpublished data). The Van Eps lab is now focusing on methods to prevent perfusion failure specifically in SLL.
What about non-ischemic energy failure?
Disturbances of energy metabolism despite adequate blood perfusion have been increasingly recognized in the pathophysiology of human sepsis-related organ failure [5] and after traumatic brain injury [6]. Although lamellar ischemia was not a feature of SRL (OF model), we have new evidence of non-ischemic oxidative energy failure later in laminitis development in this model (Figure 2B) [7], characterized by an increase in microdialysate L:P ratio (without decreased pyruvate or urea clearance), consistent with non-ischemic energy failure. Controlling this may help to prevent progression in SRL.
Therapeutic hypothermia prevents lamellar attachment failure, but why?
The van Eps laboratory has demonstrated the protective effect of continuous digital hypothermia in several experimental studies of SRL and it has translated into an effective clinical preventative. In their early studies, they found hypothermia dramatically inhibited transcription of inflammatory mediators in lamellar tissue when applied prophylactically [8]. However, while protective effects were still observed when hypothermia was applied later in the SRL model, inhibition of inflammation was no longer observed[9].
Furthermore, while the Van Eps laboratory found that hypothermia protected against the non-ischemic energy dysregulation noted in the SRL model, it also has a remarkable protective effect in the hyperinsulinemic clamp model of endocrinopathic laminitis, where there is no evidence of energy dysregulation. Thus, continued studies evaluating the specific effects of hypothermia are required to help unlock the important mechanistic pathways in SRL and endocrinopathic laminitis.
The Holy Grail- discovery of a common pathway leading to all types of laminitis?
Together with collaborators at the Ohio State University, Dr. van Eps and coworkers have generated new evidence demonstrating that lamellar signaling in all 3 models laminitis converges at the level of the mTORC1 signaling pathway, which can trigger cytoskeletal rearrangement and cell adhesion dissolution. While the exact pathways leading to mTORC1 signaling have not been fully elucidated, its induction could occur via insulin activation of IGF1-R in endocrinopathic laminitis, via IL-6 in SRL and secondary to hypoxia/ischemia in SLL. Importantly, our data indicates that mTORC1 signaling is specifically inhibited by hypothermia in SRL and endocrinopathic laminitis models, supporting this as a pharmacological target that could potentially prevent lamellar failure in different clinical forms of laminitis.
Dr. van Eps’ research is supported by the Grayson Jockey Club Research Foundation. His office may be found at Room 122, Myrin Building.
References
- Medina-Torres, C.E., Pollitt, C.C., Underwood, C., Castro-Olivera, E.M., Collins, S.N., Allavena, R.E., Richardson, D.W. and van Eps, A.W. (2014) Equine lamellar energy metabolism studied using tissue microdialysis. Vet J. 201(3):275-82.
- French, K.R. and Pollitt, C.C. (2004) Equine laminitis: glucose deprivation and MMP activation induce dermo-epidermal separation in vitro. Equine Vet J 36, 261-266.
- Medina-Torres, C.E., Underwood, C., Pollitt, C.C., Castro-Olivera, E.M., Hodson, M.P., Richardson, D.W. and van Eps, A.W. (2016) The effect of weightbearing and limb load cycling on equine lamellar perfusion and energy metabolism measured using tissue microdialysis. Equine Vet J 48, 114-119.
- Medina-Torres, C.E., Underwood, C., Pollitt, C.C., Castro-Olivera, E.M., Hodson, M.P., Richardson, D.W. and van Eps, A.W. (2016) Microdialysis measurements of lamellar perfusion and energy metabolism during the development of laminitis in the oligofructose model. Equine Vet J 48, 246-252.
- Arulkumaran, N., Deutschman, C.S., Pinsky, M.R., Zuckerbraun, B., Schumacker, P.T., Gomez, H., Gomez, A., Murray, P., Kellum, J.A. and Workgroup, A.X. (2016) Mitochondrial function in sepsis. Shock 45, 271-281.
- Gupta, D., Singla, R., Mazzeo, A.T., Schnieder, E.B., Tandon, V., Kale, S.S. and Mahapatra, A.K. (2017) Detection of metabolic pattern following decompressive craniectomy in severe traumatic brain injury: A microdialysis study. Brain Inj 31, 1660-1666.
- van eps, A.W., Poulsen, L., Belknap, J.K., Underwood, C., Schneider, X.J. and Stokes, S.M. (2017) The Effect Of Continuous Digital Hypothermia On Lamellar Energy Metabolism And Perfusion During The Development Of Laminitis In The Oligofructose Model. In: Abstracts from the 12th International Equine Colic Research Symposium July 18-20, 2017, Lexington, Kentucky. pp 38-38.
- van Eps, A.W., Leise, B.S., Watts, M., Pollitt, C.C. and Belknap, J.K. (2012) Digital hypothermia inhibits early lamellar inflammatory signalling in the oligofructose laminitis model. Equine Vet J 44, 230-237. [9] Dern, K., Watts, M., Werle, B., van Eps, A., Pollitt, C. and Belknap, J. (2017) Effect of delayed digital hypothermia on lamellar inflammatory signaling in the oligofructose laminitis model. J Vet Intern Med 31, 575-581.