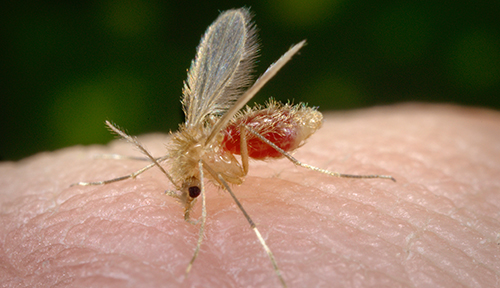
A bite from a tiny sand fly can be nearly imperceptible. But the aftermath can be devastating.
Sand flies are the vectors for leishmaniasis, a parasitic disease that affects 12 million people in the tropics, as well as dogs and other mammals. The cutaneous form of the disease causes unsightly skin ulcers that take months or even years to heal, and can progress to cause severe, disfiguring tissue damage.
“This is classically what you would call a ‘developing world disease,’ since it gets little attention,” says Dr. Phillip Scott, Vice Dean for Research and Academic Resources and Professor of Immunology at Penn Vet. “There is no vaccine and the drugs that are currently used to treat this disease are not very effective and can be quite toxic.”
For more than three decades, Scott has been partnering with scientists in Brazil and across Penn to better understand the underlying biology of the disease, with a goal of
identifying treatments that are both safe and effective. They are already moving from laboratory bench to bedside, with plans for a clinical trial.
Leishmaniasis is a particularly insidious disease because the parasite lives and replicates inside the very cells that would normally kill it: macrophages. After a bite from an infected sand fly, the parasite enters the skin; macrophages engulf the parasite and recruit T cells to help vanquish the infection. T cells, in turn, produce interferon gamma (IFNγ), a signaling molecule that instructs macrophages to kill off the pathogen.
Yet when researchers took tissue samples from people with leishmaniasis infections, they observed something puzzling. Some patients who had low levels of parasite and high levels of IFNγ, indicating that the immune system was strongly responding to the infection, still had extremely severe forms of the disease. This scenario of low parasite levels yet high IFNγ is particularly common in parts of Brazil, where Scott has worked in a clinic that sees about 1,500 leishmaniasis patients each year.
“Whenever you have this spectrum of clinical presentation, you can’t help but wonder, ‘Why does that happen?’ In my lab, we’re looking for ways to modulate the immune response to help patients avoid the most horrific forms of this disease,” Scott says.
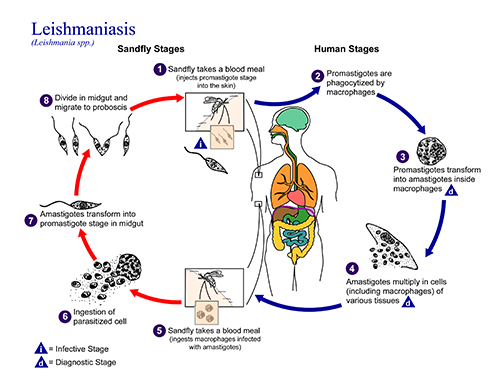
To investigate the reasons why some patients suffer such serious forms of leishmaniasis, Scott and colleagues have conducted studies in mice in his lab at Penn Vet, and in human patients at the Brazilian clinic—located in Corte de Pedra, about 3.5 hours from Brazil’s first capital, Salvador. The work, which has been supported in large part by National Institutes of Health grants awarded to Scott, has pointed to an unexpected culprit in severe cutaneous disease: a type of T cell called CD8 cells.
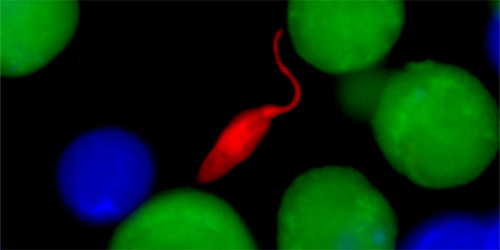
In some cases, these cells can help clear leishmania infection. But they also have a dark side. Working with postdoctoral researcher Fernanda Novais, a native Brazilian, Scott used a spinning disk confocal microscope to make videos of mouse T cells attacking the leishmania parasite. They saw that CD8 cells can actually lyse, or pop open, infected macrophages. Like poking at a hornet’s nest, the T cells end up exacerbating disease by releasing live parasites and inflammatory factors from macrophages, which can then diffuse into surrounding tissue, inflaming tissue and causing skin lesions to worsen.
With this new model of leishmaniasis pathology in hand, Scott’s lab and partners in Brazil have begun trying to understand the factors that help determine whether CD8 cells are helpful or harmful—an understanding that may one day lead to a vaccine or new therapies.
In collaboration with John Wherry, Professor in the Department of Microbiology at Penn’s Perelman School of Medicine, Scott found that having a prior viral infection
before acquiring leishmaniasis can lead to a more aggressive form of disease, likely because the immune system’s CD8 T cells have already been primed to respond to invading pathogens.
In addition, a partnership with Elizabeth Grice, Assistant Professor in the Department of Dermatology at Penn Medicine, has focused on the role of the skin microbiome— the collected community of bacteria, viruses, parasites, and other microbes—in disease progression. In mice, they’ve found that leishmaniasis infection transforms the microbiome, causing certain types of microbes to become dominant that they believe may exacerbate disease. One possibility is that using an antibiotic to reduce populations of these dominant microbes could supplement and boost the effectiveness of standard leishmaniasis therapies.
 Scott and his Brazilian colleagues are also embarking on a new project that will use blood and tissue samples from patients in Brazil to further elucidate the biology of the disease. Researchers will obtain genomic sequences from parasites, analyze the patients’ skin microbiomes, and perform transcriptional analyses on lesion tissue compared to normal tissue to see what genes are activated during
Scott and his Brazilian colleagues are also embarking on a new project that will use blood and tissue samples from patients in Brazil to further elucidate the biology of the disease. Researchers will obtain genomic sequences from parasites, analyze the patients’ skin microbiomes, and perform transcriptional analyses on lesion tissue compared to normal tissue to see what genes are activated during
infection.
“With this work, we’ll get a full picture of what’s happening in these patients,” Scott says. “One thought is that we could define biomarkers for progression to serious
disease or perhaps even drug targets.”
For now, however, researchers are hoping to leverage drugs that are already on the market to make an impact against leishmaniasis. First up is an inflammation-blocking compound called anakinra, which is widely used to treat rheumatoid arthritis. Scott’s lab has shown that anakinra is extremely effective against leishmaniasis in mice; now he is helping design a clinical trial that will enroll a handful of human patients in Brazil.
“Most tropical diseases don’t get the attention of drug companies, so if we can use a drug that is already available to effectively treat leishmaniasis, it’s a win-win,” Scott says.
Parasitic diseases have largely been wiped out in humans in the developed world. But since they remain a problem in companion animals, veterinary schools—including Penn Vet—have a strong history in the field of parasitology. Scott and his fellow scientists are continuing in this tradition, applying the concept of One Health to understand parasite biology and, perhaps one day soon, reap important benefits for human and animal health around the world.
“Working on this disease provides an opportunity to do some really interesting basic science with a great team and, what’s even more exciting, to do something that might help a lot of people,” Scott says.