VMD-PhD Student Sylvia Qu
Using engineering to bridge the gap from bench to bedside
Like many veterinary students, Feini (Sylvia) Qu grew up with a love for animals and zoology. In college, she volunteered at a lemur research center and traveled to Paraguay to study tropical birds. She also shadowed veterinarians as they worked in clinics seeing patients.
But unlike many traditional vet students, the tools and technology the clinicians used fascinated Qu almost as much as the practice of medicine itself.
“I was so excited by the instrumentation,” Qu says. “I always wanted to know how a probe or any given piece of equipment does what it does.”
That fascination led her to Penn Vet’s VMD-PhD Program, and to pursue interdisciplinary research into tissue engineering to create a better approach to connective tissue repair surgeries—for both people and pets.
Qu majored in biomedical engineering as an undergraduate at Duke University and entered Penn Vet’s dual-degree program with a good sense of what she wanted to pursue for her PhD. While some combined-degree students rotate through different labs during the summers between their first years of veterinary school, she spent much of her time in the lab of Dr. Robert Mauck, Associate Professor of Orthopaedic Surgery and Bioengineering, with appointments in the Perelman School of Medicine and the School of Engineering and Applied Science.
“I came in with an interest in tissue engineering and orthopedics,” she says. “I wanted to be the person with a knowledge of both the engineering side and the veterinary side to help with making the bench-to-bedside transition.”
To do so, preclinical studies in large animal models are typically a necessary step, and were a major part of Qu’s dissertation research, which focused on strategies to repair injuries to connective tissues such as tendons, ligaments, and cartilage.
In particular, Qu zeroed in on attempts to repair the meniscus, a rubbery, C-shaped piece of dense tissue known as brocartilage that cushions the knee joint. Meniscus tears are a common injury, but very difficult to repair because only the outer third of the tissue receives significant blood flow. Injuries that are complex or that affect the inner part of the meniscus are treated by surgery to cut out and remove the torn portion, rather than attempt a repair.
For decades, researchers have attempted to encourage better healing by designing scaffolds on which new cells could accumulate, but efforts have fallen short. Qu, collaborating with Mauck and postdoctoral researcher Dr. Matthew Fisher, worked on an innovative approach to build a nanoscale scaffold that would not only direct cells where to go and how to be structured appropriately, but would also influence tissue at the repair site to increase the odds of a successful healing process.
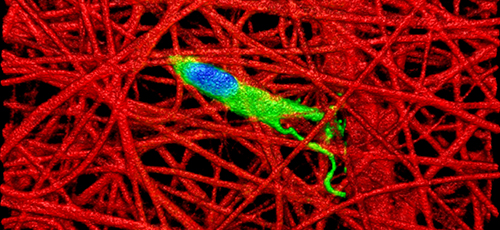 Her work was based on the recognition that fetal connective tissue is better at healing than adult tissue. Using advanced imaging technology, Qu found that this enhanced healing capability may be due to the fact that, whereas adult connective tissues such as the meniscus are stiff and dense so as to bear up under heavy loads, fetal tissue is less dense and softer. This “looser” structure is more conducive to allowing cells to migrate through a tissue to a wound site and promote healing.
Her work was based on the recognition that fetal connective tissue is better at healing than adult tissue. Using advanced imaging technology, Qu found that this enhanced healing capability may be due to the fact that, whereas adult connective tissues such as the meniscus are stiff and dense so as to bear up under heavy loads, fetal tissue is less dense and softer. This “looser” structure is more conducive to allowing cells to migrate through a tissue to a wound site and promote healing.
What if, the researchers wondered, they could somehow revert adult tissue to a more fetal-like state to improve healing?
“The idea is to degrade the tissue next to the wound site so it’s looser and easier for cells to migrate in,” Qu says.
Qu and colleagues first worked in vitro to examine whether the collagen-degrading enzyme collagenase effectively digested the extracellular matrix of adult cow meniscus tissue. They found that, indeed, the matrix density decreased and the number of cells increased when incubated with the enzyme.