 Dr. Rumela Chakrabarti (left) with her lab, including Sushil Kumar (far right).
Dr. Rumela Chakrabarti (left) with her lab, including Sushil Kumar (far right).
About 15 percent of breast cancers are classified as triple-negative, lacking receptors for estrogen, progesterone, and Her2. These cancers do not respond to targeted hormonal therapies, and they tend to be particularly aggressive, often resisting systemic chemotherapy and metastasizing to other tissues.
Researchers had observed that triple-negative breast cancer (TNBC) patients who had higher numbers of a type of immune cell called myeloid-derived immunosuppressor cells (MDSCs) in their bloodstream had poorer outcomes. But until now it wasn’t clear how MDSCs are recruited to the primary breast tumor, and how they contributed to its progression and spread.
 Dr. Rumela Chakrabarti
Dr. Rumela Chakrabarti
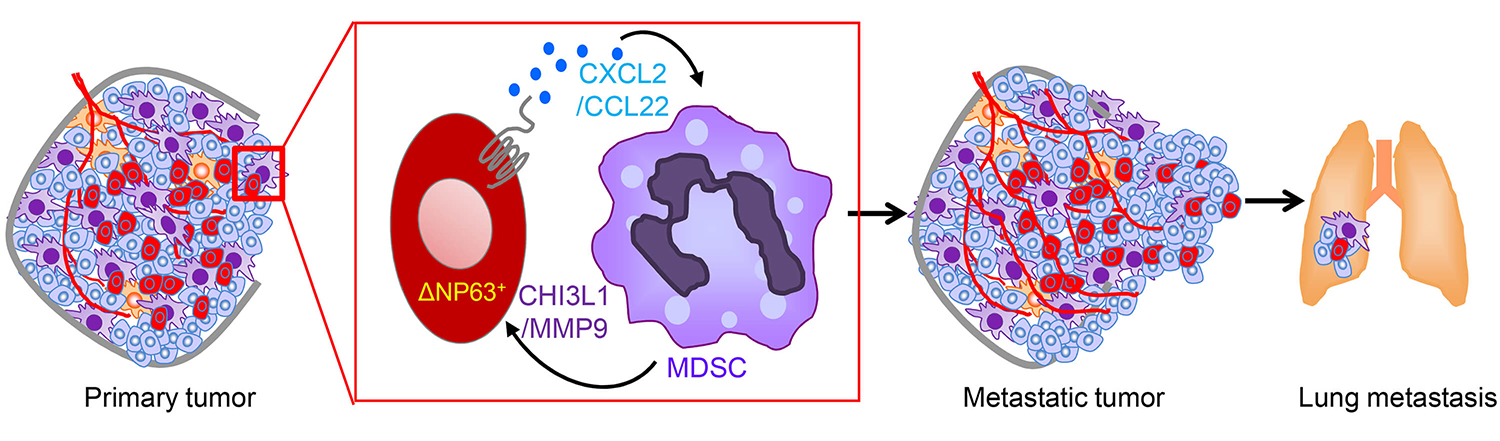
A new report led by the School of Veterinary Medicine’s Rumela Chakrabarti, an assistant professor of biomedical sciences, and Sushil Kumar, a postdoctoral researcher in Chakrabarti’s lab, fills in crucial details about the connection between MDSCs and aggressive disease. In the Journal of Clinical Investigation, Chakrabarti, Kumar, and colleagues identify a protein, deltaNp63, on tumor cells that directs MDSCs to the tumor and metastatic sites, where they promote tumor growth and metastasis. Blocking either this protein or the MDSCs themselves reduced tumor growth and metastasis in a mouse model of TNBC.
“We’re excited because we think our findings could make a big difference for triple-negative breast cancer patients,” says Chakrabarti. “Not only can deltaNp63 be used as a biomarker to help personalize treatment regimens, but targeting it may also provide an additive treatment for triple-negative breast cancer, in addition to chemotherapy and radiation.”
Earlier studies by Chakrabarti and colleagues showed that increased levels of deltaNp63 were linked with breast cancer initiation. In the current work, her team found deltaNp3 was elevated in samples of TNBC patient’s primary tumors, as were numbers of MDSCs.
 Immune cells called myeloid-derived immunosuppressor cells (MDSCs) play a key role in the progression and aggressiveness of triple-negative breast cancers. Blocking them could offer a therapeutic target in the disease, which notoriously resists many standard treatments.
Immune cells called myeloid-derived immunosuppressor cells (MDSCs) play a key role in the progression and aggressiveness of triple-negative breast cancers. Blocking them could offer a therapeutic target in the disease, which notoriously resists many standard treatments.
Intrigued, Chakrabarti, Kumar, and their coauthors used multiple mouse models and tissue transplants to see how manipulating the level of deltaNp63 affected the behavior of cancer. They found lower levels corresponded with less metastasis to distant tissues. In addition, knocking down levels of deltaNp63 made the tumors much less aggressive, and it reduced numbers of MDSCs recruited to the tumor but not other immune cell types.
The researchers confirmed the relationship between deltaNp63 and MDSCs, showing that blocking two signaling molecules, CXCL2 and CCL22, activated by the protein reduced metastasis and blood-vessel growth associated with tumor growth, while increasing levels of these signaling molecules caused MDSCs to boost the secretion of pro-tumor growth factors.
“How are the immune cells helping cancer cells?” Chakrabarti says. “It seems they are helping cancer stem cells grow faster.” Cancer stem cells can give rise to all the other cells in a tumor, just as normal stem cells can differentiate into other cell types. These cells are resistant to chemotherapy and radiation, which may explain why triple-negative breast cancer patients don’t respond to therapy, Chakrabarti says. “We propose that it’s these immune cells [MDSCs] that are nurturing the cancer stem cells.”
The research team used small molecules to inhibit CXCL2 and CCL22 in human TNBC cell lines, as well as in a mouse model of TNBC, a blockade that significant reduced levels of MDSCs moving to the primary tumor and that substantially lowered signs of metastasis.
Chakrabarti believes that a drug that zeroes in on MDSCs could fill a gap in triple-negative breast cancer treatment. Offered in conjunction with more general therapies such as chemotherapy and radiation, it may give patients an option that is more tailored to their cancer. Her lab is now working with animal models and cell lines derived from breast-cancer patients to test this combination approach to treatment.
In addition to Chakrabarti and Kumar, the study’s coauthors were Penn Vet’s David W. Wilkes, Nina Samuel, Mario Andrés Blanco; the Perelman School of Medicine’s Anupma Nayak; The Wistar Institute’s Dmitry Gabrilovich and Kevin Alicea-Torres, also of Penn Medicine; and the State University of New York’s Christian Gluck and Satrajit Sinha.
The work was supported by the American Cancer Society, McCabe Fund, Breakthrough Bike Challenge of the Abramson Cancer Center, and National Cancer Institute (Grant CA193661).