
A leading global cause of diarrheal disease and malnutrition in young children, the Cryptosporidium parasite can outsmart the human immune system in order to invade and take over its host cell functions.
In a new study, a team led by researchers from Penn’s School of Veterinary Medicine used powerful video microscopy to track one of the earliest steps of infection, invasion of the cells that line the intestine. This process might one day be the target of a vaccine or therapy for a serious illness for which effective therapy and prevention are currently lacking.
Prior to this work, it was anticipated that the parasites likely delivered proteins into host cells before they invade, but the proteins’ identity had remained a mystery. The new findings, published in the journal Cell Host & Microbe, identify how one injected protein interacts with a host protein, initiating a molecular battle that sets the stage for the parasite to invade more efficiently.
 Dr. Boris Striepen
Dr. Boris Striepen
“We were interested in two questions,” says Boris Striepen, senior author on the study and a Penn Vet professor. “One is, How do these parasites that live within our cells get in? And the second is, How do they force the host to dramatically remodel the cell for the parasite’s gain?”
The study relied on an advanced microscopy technique developed by Amandine Guérin, a postdoctoral fellow in Striepen’s lab and first author on the paper, to unlock secrets of the process of Cryptosporidium infection, a process that has until now been poorly understood.
“We established a system in which host and parasite interact under high-powered microscope imaging at high frequency, which allowed us to record the process of invasion,” says Guérin. “Doing that, we could see proteins of both parasite and host, using fluorescent labels, and track them throughout their complex interaction.”
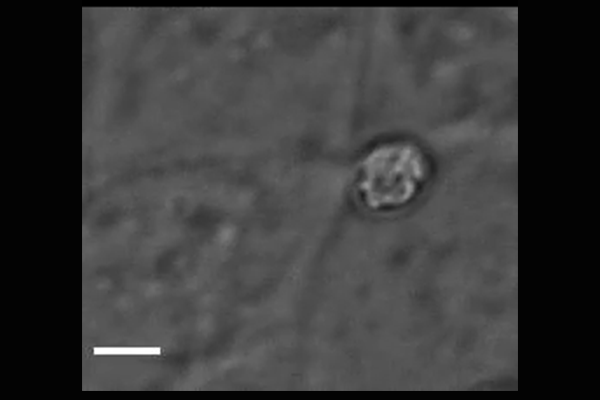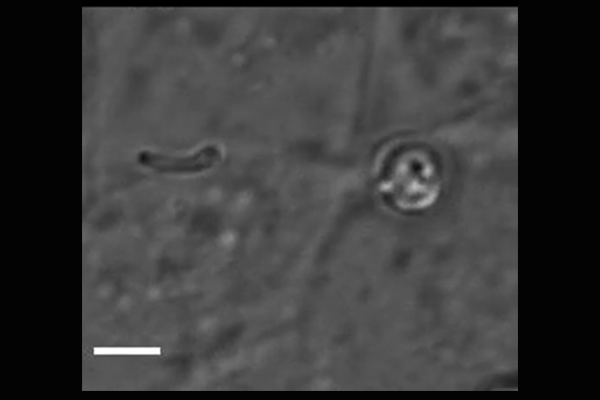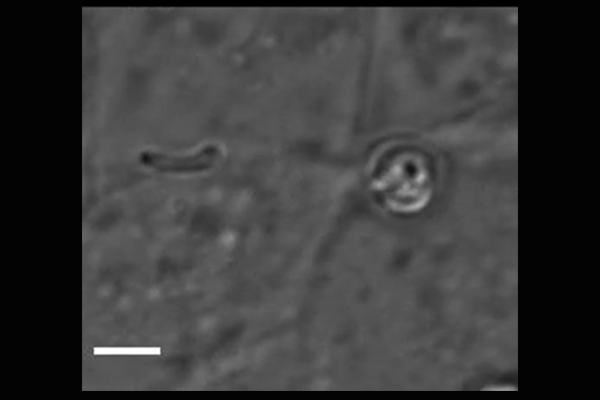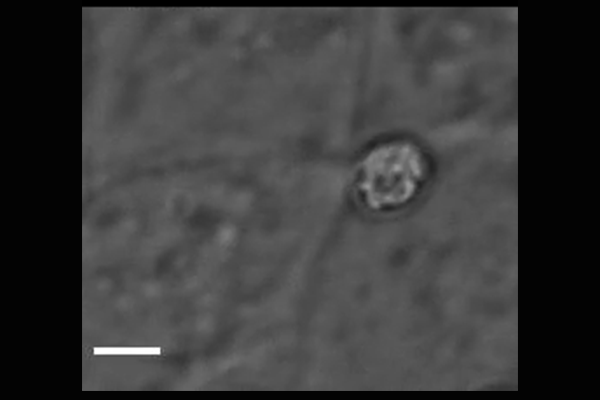 Cryptosporidium parasites emerge from the spore-like oocyst and invade a human cell. Powerful microscopy enabled Penn Vet scientists to track and identify some of the molecular players in the first stages of
Cryptosporidium parasites emerge from the spore-like oocyst and invade a human cell. Powerful microscopy enabled Penn Vet scientists to track and identify some of the molecular players in the first stages of
parasite invasion. (Image: Amandine Guérin)
By genetically attaching green fluorescent labels to different host cell proteins and red labels to the parasite itself, they could see how, by contorting itself, the parasite forces its way into the host cell. This process took about two minutes and resulted in the reshaping of the host’s membrane and actin cell skeleton.
From other studies, the researchers understood that the parasite likely uses an injection device called the rhoptry to trigger these changes in its host. To uncover the identity of these injected factors, they used experimental and computational approaches to screen the more than 3,500 parasite proteins for likely candidates. The team then looked closer at individual proteins to find out when they are injected and which components of the host they specifically manipulate.
By tagging several different rhoptry proteins with labels they could later visualize, the researchers observed that, while delivered together, the proteins moved to different places within the host cell; some stayed adjacent to the membrane, while others dispersed through the cell or bound to its skeleton.
“Essentially Amandine found that discharge occurs in the moment the parasite first touches the host cell, and this is the start signal that begins this whole invasion,” says Striepen. “It’s like the Normandy landing: Troops arrive together in landing craft, but once they are on the beach they immediately disperse to pursue different strategic objectives.”
To learn more about these different functions, the researchers engineered human as well as yeast cells to make one of the rhoptry proteins, ROP1, in the absence of Cryptosporidium. They found that when ROP1 was delivered into the host, it interacted with the host protein LMO7, which other studies have found to play a role in cell-to-cell adhesion and the organization of the cell skeleton. In cell cultures and mice infected with Cryptosporidium, they observed ROP1 and LMO7 together at the surface of infected cells.
The researchers then manipulated both proteins to determine the impact on infection. When mice lacking LM07 were infected with the parasite, their infection burden was higher than in normal mice. In contrast, in mice infected with parasites lacking ROP1, the parasite burden appeared reduced compared to normal mice.
“Infection wasn’t abolished, but it was diminished,” Striepen says. “These two proteins, in the host and in the parasite, appear to be in an antagonistic relationship with one another.”
In future work, the researchers hope to understand in more detail how ROP1 and other rhoptry proteins are able to change host cell functions. And they also aim to delve further into the co-evolution of parasite attack strategies with host defense strategies.
“By injecting proteins into the host, the parasite is able to effect these changes but is also makes itself very visible to the immune system,” Striepen says. “That’s a trade-off, and it could also mean these proteins could eventually be promising candidates to target with a vaccine.”
Amandine Guérin is a postdoctoral fellow in the University of Pennsylvania School of Veterinary Medicine.
Boris Striepen is the Mark Whittier and Lila Griswold Allam Professor of Microbiology and Immunology in Penn’s School of Veterinary Medicine.
Guérin and Striepen’s coauthors were Emily M. Kugler of Penn Vet, Laurance Berry of the University of Montpellier, Nathan H. Roy and Janis K. Burkhardt of Penn’s Perelman School of Medicine and the Children’s Hospital of Philadelphia, and Jung-Bum Shin of the University of Virginia.
The study was supported in part by the National Institutes of Health (grants AI112427 and AI148249), the Pennsylvania Department of Health, and the European Molecular Biology Organization