Genetic basis and molecular mechanisms of inherited retinal diseases
1. Retinal diseases with multigenic etiology
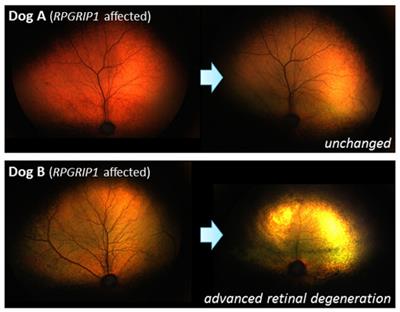 Clinically, there are variations in retinal disease expression such as the onset of impaired vision, progression, and severity even among individual animals and patients sharing the same disease mutation. While most inherited retinal diseases (IRDs) have been thought as Mendelian or monogenic disorders, the involvement of multiple gene mutations as well as genetic modifiers affecting the disease outcome has increasingly been recognized. However, searching for and validating modifiers in a genetically diverse human populations is challenging. One of our research interest is cone-rod dystrophy (cord1) in dogs, previously associated with a mutation in RPGRIP1. Following the identification of the first genetic modifier, we continue to investigate and tease out the multiple molecular players associated with this condition.
Clinically, there are variations in retinal disease expression such as the onset of impaired vision, progression, and severity even among individual animals and patients sharing the same disease mutation. While most inherited retinal diseases (IRDs) have been thought as Mendelian or monogenic disorders, the involvement of multiple gene mutations as well as genetic modifiers affecting the disease outcome has increasingly been recognized. However, searching for and validating modifiers in a genetically diverse human populations is challenging. One of our research interest is cone-rod dystrophy (cord1) in dogs, previously associated with a mutation in RPGRIP1. Following the identification of the first genetic modifier, we continue to investigate and tease out the multiple molecular players associated with this condition.
Please see Participation to learn more about the ongoing study on progressive retinal atrophy (PRA) in the English Springer Spaniels.
2. Gene therapy to target the secondary neurons in the retina
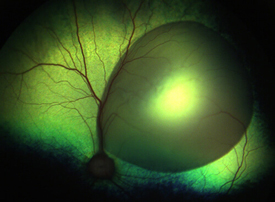 We have characterized, both clinically and molecularly, congenital stationary night blindness (Schubert-Bornstein type) in a family of dogs. Affected dogs have dysfunction of the secondary neurons (ON-bipolar cells) of the retina. While retinal gene therapies that are commercialized or under development target the primary site of vision (photoreceptors and the retinal pigmented epithelia), we are therapeutically targeting these secondary neurons.
We have characterized, both clinically and molecularly, congenital stationary night blindness (Schubert-Bornstein type) in a family of dogs. Affected dogs have dysfunction of the secondary neurons (ON-bipolar cells) of the retina. While retinal gene therapies that are commercialized or under development target the primary site of vision (photoreceptors and the retinal pigmented epithelia), we are therapeutically targeting these secondary neurons.
3. Photoreceptor degeneration associated with ciliopathy
The connecting cilium of the photoreceptors bridges the inner and outer segments, playing a vital role in normal organization and function of both rods and cones. Among the dogs affected with retinal degeneration, we have identified mutations in genes that are important in the connecting cilium. We are characterizing these still largely unknown molecules along with their interacting partners with the aim to identify therapeutic targets.
Gene therapy to reverse corneal clouding in patients with lysosomal storage disease
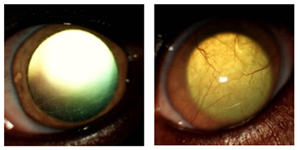 In collaboration with Dr. Matthew Hirsch at the University of North Carolina-Chapel Hill, we are pioneering to establish a safe, effective, and sustainable AAV gene therapy in preventing and reversing corneal clouding in mucopolysaccharidosis I (MPS1) patients. In preliminary studies , using the MPS1 canine model, we have shown that intrastromal AAV-IDUA injection is effective in preventing and reversing corneal clouding.
In collaboration with Dr. Matthew Hirsch at the University of North Carolina-Chapel Hill, we are pioneering to establish a safe, effective, and sustainable AAV gene therapy in preventing and reversing corneal clouding in mucopolysaccharidosis I (MPS1) patients. In preliminary studies , using the MPS1 canine model, we have shown that intrastromal AAV-IDUA injection is effective in preventing and reversing corneal clouding.
 Keiko Miyadera, DVM, PhD, DACVO| Principal Investigator
Keiko Miyadera, DVM, PhD, DACVO| Principal Investigator
Dr. Miyadera is a clinician scientist with a passion for canine genetics. As a veterinary ophthalmologist, she cares deeply for vision and comfort of the animal eye; as a researcher, she investigates the underlying genetics of hereditary eye diseases in dogs, and pursues new diagnostics (DNA test) and therapies (gene therapy).
She studied veterinary medicine at the University of Tokyo, Japan (DVM) and molecular biology at the University of Cambridge, UK (PhD) before moving to Philadelphia. At Penn, she pursued further research training with Dr. Gustavo Aguirre and completed a veterinary ophthalmology residency (Diplomate of the American College of Veterinary Ophthalmology) before starting her own research lab.
Dr. Miyadera believes that by studying the molecular basis of hereditary eye diseases in dogs, we can better understand the equivalent vision-threatening diseases in people and develop targeted therapies to prevent and treat vision-threatening diseases.
Dr. Miyadera is a member of the Division of ExpeRTs at Penn Vet.
Lab Members & Collaborators
- Jessica Niggel, M.Sc.
- Jennifer Kwok, DVM
- Leonardo Murgiano, PhD
- Kumiko Kato, DVM PhD DAiCVO
Past Members
- Kei Takahashi, BS RPh, PhD (Penn Vet)
- Alexa Grey, DVM (Cornell University)
- Yu Sato, DVM (University of Florida)
- Emma Ozdogan (New York University)
- Karolina Roszak, DVM (UC Davis)
- Rueben G. Das, PhD (Penn Medicine)
- Kendall Carlin, DVM (Tufts University)
- Luis Felipe Marinho, DVM PhD
- Courtney Spector, BSc
Genetics of Progressive Retinal Atrophy (PRA) in English Springer Spaniels
Enrollment Forms
- For sending blood samples and eye exam reports (PDF)
- For participation in eye screening exams at national and regional specialties (PDF)
English Springer Spaniel PRA Genetics Study
If you have a dog with DNA banked at the University of Missouri, please fill out our study form to update the information critical for the PRA genetics study.
Fill out the PRA Genetics Study Form Now
Background
 A mutation in the gene RPGRIP1 has previously been associated with a blinding retinal disease (PRA, cord1, or crd4) in the Dachshunds. Later, the same mutation was found to be relatively common in the English Springer Spaniels (ESS). This is despite the low incidence of clinically affected ESS. Due to this discrepancy, the significance of the RPGRIP1 DNA testing in ESS has been ambiguous. The purpose of this study is to clarify the relation between the RPGRIP1 mutation and retinal diseases in ESS. We suspect that multiple factors are involved, including RPGRIP1. Our goal is to tease out the individual factors and establish a reliable set of DNA tests that allows accurate prediction of the course of disease.
A mutation in the gene RPGRIP1 has previously been associated with a blinding retinal disease (PRA, cord1, or crd4) in the Dachshunds. Later, the same mutation was found to be relatively common in the English Springer Spaniels (ESS). This is despite the low incidence of clinically affected ESS. Due to this discrepancy, the significance of the RPGRIP1 DNA testing in ESS has been ambiguous. The purpose of this study is to clarify the relation between the RPGRIP1 mutation and retinal diseases in ESS. We suspect that multiple factors are involved, including RPGRIP1. Our goal is to tease out the individual factors and establish a reliable set of DNA tests that allows accurate prediction of the course of disease.
Do you own an English Springer Spaniel?
We are calling for participation if your ESS has either been:
- Diagnosed or suspected with retinal degeneration (PRA)
- DNA tested as affected for the RPGRIP1 mutation
- Related to any ESSs meeting one of the above criteria
We are also recruiting any other ESSs that can take part in eye examination (please contact us for details) to enroll clinically and genetically ‘healthy’ dogs to participate in the study as controls. They are retinal changes associated with the disease that may not be recognized clinically, and we encourage any ESSs that can to take advantage of the opportunity and to participate.
What is needed for participation?
We will ask:
- Blood samples (drawn by your veterinarian or by us)
- Eye examination by qualified veterinary ophthalmologist (at Penn Vet or elsewhere)
- Copy of pedigree (if available)
This study is funded by the American Kennel Club Canine Health Foundation and supported by the English Springer Spaniel Field Trial Association Foundation.
Genetics of primary glaucoma in Shiba-Inu dogs
Primary glaucoma is a devastating eye disease in Shiba-Inu Dogs that almost inevitably leads to vision loss while causing significant discomfort. Affected eyes often require eye removal surgery due to difficulties controlling the disease. Primary glaucoma in Shiba-Inus is considered genetic, caused by defects in the drainage pathway within the eye, leading to pressure build-up over time.
At Penn Vet, we are in the process of developing a study aimed at searching for genetic markers that can help determine the risk of developing glaucoma in Shiba-Inus. Once markers that correlate with the disease are identified, DNA tests can be established. DNA tests are useful in determining the optimal breeding for reducing the risk of glaucoma. It also allows identifying at-risk dogs that could benefit from starting preventative therapies sooner.
We are collecting blood samples from Shiba-Inus that have been examined by an ophthalmologist and had gonioscopy performed. Gonioscopy is a test that evaluates the drainage angle within the eye that is associated with the risk of glaucoma.
We are now enrolling:
- A. Shibas that have been diagnosed with glaucoma
- B. Healthy Shibas that have had gonioscopy performed
Current requirements for enrollment are:
- Blood samples (cheek swabs may be accepted, but we strongly prefer and appreciate blood samples for maximal impactful in the study)
- Submission form (PDF)
- Copy of eye exam records
- Copy of pedigree (if available)
Detailed instructions can be found in the submission form.
Journal Articles
Murgiano L, Becker D, Spector C, Carlin K, Santana E, Niggel JK, Jagannathan V, Leeb T, Pearce-Kelling S, Aguirre GD, Miyadera K. CCDC66 frameshift variant associated with a new form of early-onset progressive retinal atrophy in Portuguese Water Dogs. Scientific Reports. 10(1):21162. 2020.
Miyadera K, Conatser L, Llanga TA, Carlin K, O'Donnell P, Bagel J, Song L, Kurtzberg J, Samulski RJ, Gilger B, Hirsch ML. Intrastromal Gene Therapy Prevents and Reverses Advanced Corneal Clouding in a Canine Model of Mucopolysaccharidosis I. Molecular Therapy. 28(6):1455-1463. 2020.
Das RG, Becker D, Jagannathan V, Goldstein O, Santana E, Carlin K, Sudharsan R, Leeb T, Nishizawa Y, Kondo M, Aguirre GD, Miyadera K. Genome-wide association study and whole-genome sequencing identify a deletion in LRIT3 associated with canine congenital stationary night blindness. Scientific Reports. 9(1):14166. 2019.
Miyadera K. Mapping of Canine Models of Inherited Retinal Diseases. Advances in Experimental Medicine and Biology. 1074:257-264. 2018.
Das Rueben G, Marinho Felipe Pompeo, Iwabe Simone, Santana Evelyn, McDaid Kendra Sierra, Aguirre Gustavo D, Miyadera Keiko Variabilities in retinal function and structure in a canine model of cone-rod dystrophy associated with RPGRIP1 support multigenic etiology. Scientific reports 7: 12823, 2017.
Forman Oliver P, Hitti Rebekkah J, Boursnell Mike, Miyadera Keiko, Sargan David, Mellersh Cathryn Canine genome assembly correction facilitates identification of a MAP9 deletion as a potential age of onset modifier for RPGRIP1-associated canine retinal degeneration. Mammalian genome : official journal of the International Mammalian Genome Society 27: 237-45, 2016.
Tanaka N, Dutrow EV, Miyadera K, Delemotte L, MacDermaid CM, Reinstein SL, Crumley WR, Dixon CJ, Casal ML, Klein ML, Aguirre GD, Tanaka JC, Guziewicz KE Canine CNGA3 Gene Mutations Provide Novel Insights into Human Achromatopsia-Associated Channelopathies and Treatment. PloS one 10: e0138943, 2015.
Kondo M, Das G, Imai R, Santana E, Nakashita T, Imawaka M, Ueda K, Ohtsuka H, Sakai K, Aihara T, Kato K, Sugimoto M, Ueno S, Nishizawa Y, Aguirre GD, Miyadera K A Naturally Occurring Canine Model of Autosomal Recessive Congenital Stationary Night Blindness. PloS one 10: e0137072, 2015.
Miyadera K Inherited retinal diseases in dogs: advances in gene/mutation discovery. Dobutsu iden ikushu kenkyu = Journal of animal genetics 42: 79-89, 2014.
Miyadera K, Kato K, Boursnell M, Mellersh CS, Sargan DR Genome-wide association study in RPGRIP1(-/-) dogs identifies a modifier locus that determines the onset of retinal degeneration. Mammalian genome : official journal of the International Mammalian Genome Society 23: 212-23, 2012.
Miyadera K, Acland GM, Aguirre GD Genetic and phenotypic variations of inherited retinal diseases in dogs: the power of within- and across-breed studies. Mammalian genome : official journal of the International Mammalian Genome Society 23: 40-61, 2012.
Miyadera K, Brierley I, Aguirre-Hernández J, Mellersh CS, Sargan DR Multiple mechanisms contribute to leakiness of a frameshift mutation in canine cone-rod dystrophy. PloS one 7: e51598, 2012.
Miyadera K, Kato K, Aguirre-Hernández J, Tokuriki T, Morimoto K, Busse C, Barnett K, Holmes N, Ogawa H, Sasaki N, Mellersh CS, Sargan DR Phenotypic variation and genotype-phenotype discordance in canine cone-rod dystrophy with an RPGRIP1 mutation. Molecular vision 15: 2287-305, 2009.
News
Watching Sheeba’s Eyes (Penn Today) - 4/19/2019
Dr. Miyadera was inviter to speak at the Dog Genetics Forum at the University of Tokyo, Japan - 3/9/2019
Dr. Miyadera was interviewed in the Ryan Report of Penn Vet – 2/9/2016
Penn Vet Team Identifies a Form of Congenital Night Blindness in Dogs (Penn Vet Press Release) - 9/14/2015