
Dr. Rumela Chakrabarti is an assistant professor (tenure-track) in the Department of Biomedical Sciences. Dr. Chakrabarti attended Pune University, India, receiving her B.S. in Zoology in 1999 and an M.S. in Genetics in 2001. She then moved to the United States and received her Ph.D. in Biomedical sciences in 2007 from Kent State University, Ohio, where she studied the function of phosphatases in sperm formation and motility. Being trained as a developmental biologist during her Ph.D., she next joined the State University of New York at Buffalo, New York as a post-doctoral fellow where she investigated the function of transcription factors in dictating the cell fate of stem and progenitor cells in mammary gland development. Her successful initial studies on mammary stem cells then inspired her to move to Princeton University, New Jersey for additional postdoctoral training where she extended and integrated her expertise and knowledge in normal mammary gland biology to understand the role of stem cells in breast cancer initiation, progression and metastasis. Dr. Chakrabarti is interested in the fundamental question of how breast cancer originates from normal cells and how molecular events in early tumorigenesis influence the course of disease, including metastatic progression. Additionally, she is interested in how cell signaling between breast cancer cells and their microenvironment dictates the fate of these cancer cells.
Cell fate regulators of normal mammary gland development in breast cancer
Breast cancer remains a major health threat due to therapeutic challenges driven by both its heterogeneity and metastatic recurrence. Genes and signaling pathways that control normal mammary epithelial differentiation influence the formation and evolution of different subtypes of breast cancer. During her postdoctoral work, Dr. Chakrabarti showed that the transcription factor (TF) Elf5, an ETS family TF, is a master regulator for the establishment and maintenance of the luminal epithelial cells in the mammary gland that produce milk during pregnancy (1).
Conditional knockout of Elf5 completely abolishes lactation due to reduced differentiation of mature luminal cells, accompanied by an accumulation of luminal progenitors. Dr. Chakrabarti also identified a novel role for Elf5 in inhibiting mammary stem cell activity, thereby promoting luminal differentiation during normal mammary gland development.
Notably, these studies identified Elf5 as a key specifier of alveolar fate, acting through direct repression of Notch signaling(2). In addition to demonstrating a role for Elf5 in influencing normal mammary cell fate, Dr. Chakrabarti discovered a novel function for Elf5 in inhibiting the epithelial mesenchymal transition (EMT) that occurs in both physiological and pathological contexts, and plays an important role in metastasis of the basal subset of breast cancers (3).
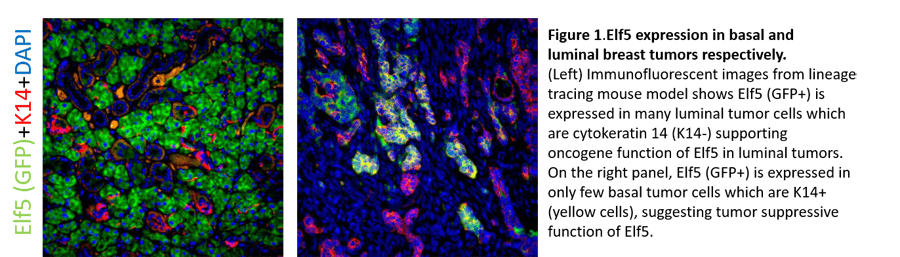
Mechanistically, her group observed that Elf5 expression represses the expression of several EMT-promoting transcription factors, including TWIST1, ZEB1/2, and SLUG. Dr. Chakrabarti is currently determining if Elf5 is also important for earlier changes in breast tumor development and progression using state-of-the art techniques, including conditional knockout mice and lineage tracing Elf5 reporter models. Lineage tracing data and functional studies suggest that Elf5 functions early during cancer progression and may dictate tumor metabolism (manuscript in preparation). Finally, her recent data suggest that Elf5 functions in a tumor subtype-specific manner to drive either tumor promotion or suppression highlights the need to understand the specific effects of targeted therapy in individual breast cancer subtypes to identify effective chemotherapeutics (Figure 1).
As noted above, Notch signaling is important mammary gland development. However, Notch signaling also drives breast cancer progression and is therefore an attractive therapeutic target. Inhibitors blocking signaling of all four Notch receptors reduce tumor growth, but their broad target specificity and associated gastrointestinal toxicity limits their therapeutic utility. Although therapies targeting specific Notch receptors or ligands are still lacking, preliminary studies from Dr. Chakrabarti’s group indicates that Notch signaling mediated by the ligand Dll1 promotes mammary stem cells activity by interacting with neighboring macrophages through a unique pathway involving Notch-Wnt crosstalk (manuscript under final revision). In breast cancer, her group found that, although Dll1 does not influence basal breast cancer, it promotes luminal breast cancer growth and metastasis, suggesting a unique subtype specific function, which is also dependent on Estrogen signaling (manuscript submitted). Dr. Chakrabarti’s laboratory proposes to use lineage tracing models and knockout mice model to understand the fate of Dll1+ cells in luminal breast cancer and will dissect the mechanistic basis for Dll1-mediated promotion of luminal breast cancer to aid in development of safer ligand based therapy of breast cancer patients.
Recruitment of immune cells by cancer cells in breast cancer
Triple-negative breast cancer (TNBC) represents about 20% of all breast cancers and is a particularly aggressive subtype with increased metastasis and mortality and limited treatment options. Dr. Chakrabarti has previously shown that a transcription factor, ΔNp63 is important for tumor initiation of TNBCs by regulating Wnt signaling and her studies demonstrate that ΔNp63 regulates both normal mammary stem cells and tumor initiating cells in TNBC through regulation of the Wnt receptor Fzd7. Recent studies involving Dr. Sushil Kumar in her group now show that high levels of the transcription factor ΔNp63 are associated with increased metastasis of TNBC.
Notably, increased expression of ΔNp63 is associated with increased numbers of myeloid-derived tumor suppressor cells (MDSCs), an immune cell subset associated with tumor progression and metastasis in several cancers including breast cancer. Dr. Chakrabarti’s laboratory found that ΔNp63 promotes recruitment of MDSCs to the tumor microenvironment by upregulating chemokine expression (manuscript submitted). Moreover, they found that blocking chemokine signaling reduced MDSC recruitment and metastasis, highlighting a novel crosstalk between ΔNp63+ TNBC cells and MDSCs driving tumor progression. Using these findings as a starting point, her lab is currently validating the efficacy of combined immunotherapy/chemotherapy strategies that they hope will improve the prognosis for TNBC patients in the not too distant future.
Dr. Chakrabarti’s research is funded by the NIH/NCI K22 (K22CA193661-01) grant, Breakthrough Bike Challenge award and McCabe award from Abramson Cancer Center. Her laboratory is located in the Hill Pavilion, H432 and her office is in Hill Pavilion H411.
References
- Choi YS, Chakrabarti R, Escamilla-Hernandez R, Sinha S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev Biol 2009;329:227-41
- Chakrabarti R, Wei Y, Romano RA, DeCoste C, Kang Y, Sinha S. Elf5 regulates mammary gland stem/progenitor cell fate by influencing notch signaling. Stem Cells 2012;30:1496-508
- Chakrabarti R, Hwang J, Andres Blanco M, Wei Y, Lukacisin M, Romano RA, et al. Elf5 inhibits the epithelial-mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nature cell biology 2012;14:1212-22