Dr. Marie-Eve Fecteau is an associate professor of Farm Animal Medicine and Surgery (Clinician Educator track) in the Department of Clinical Studies-New Bolton Center. Dr. Fecteau received her veterinary degree from the Université de Montréal in 1999 and subsequently completed a large animal rotating internship at the same institution. Following her internship, she continued to work there for one year as a staff clinician in their farm animal ambulatory service, practicing mostly on dairy cattle. Dr. Fecteau then entered a Large Animal Internal Medicine-Food Animal Emphasis residency program at the School of Veterinary Medicine of the University of California-Davis and became a diplomate of the college in 2004. Later that same year, Dr. Fecteau joined the PennVet Faculty as an Assistant Professor of Farm Animal Medicine and Surgery, and subsequently joined the Johne’s Laboratory under the mentorship of Drs. Robert Whitlock and Raymond Sweeney. Dr. Fecteau’s research interests are centered on the pathophysiology and prevention of paratuberculosis in cattle, and the pathogen’s influence on the host’s intestinal microbiome.
Background
Paratuberculosis (or Johne’s Disease (JD)) is a chronic gastrointestinal disease of cattle caused by an infection with Mycobacterium avium subsp. paratuberculosis (MAP). Infection with MAP results in inflammation of the intestinal lining, chronic diarrhea, weight loss, and is ultimately fatal. Most animals become infected during the neonatal period but due to a long incubation period (up to 5-7 years), the majority (95%) of infected animals do not show signs of illness. The absence of clinical signs in the early stages of infection, along with the lack of completely reliable diagnostic methods in the preclinical stages of the disease, and the extreme resistance of the organism in the environment, make prevention and control of MAP infection very difficult. Moreover, there are no drugs currently approved for the prevention or treatment of paratuberculosis in cattle in the United States, and vaccination is not fully protective.
Protecting the calf
As most calves become infected with MAP very early in life, current control programs focus on preventing transmission from adult cattle shedding MAP organisms in feces to young replacement stock on the farm. However, when a herd is heavily infected with MAP, preventing exposure of young stock to the MAP organisms is nearly impossible. Some of Dr. Fecteau’s earlier work focused on the chemoprophylaxis of MAP infection in neonatal calves, using gallium (Ga) compounds. Ga is a trivalent semi-metal that shares many similarities with ferric iron and functions as an iron mimic. In susceptible organisms, Ga substitutes for ferric iron in many cellular metabolic pathways and disrupts them. Fecteau initially demonstrated the antimicrobial efficacy of Ga against 10 different MAP isolates in vitro.1 In a follow up experiment, Fecteau and colleagues demonstrated the safety of two Ga compounds (Ga nitrate and Ga maltolate) and compared the serum and tissue concentrations of the two compounds following oral administration.2 Lastly, they demonstrated that in experimentally infected neonatal calves, Ga treatment was associated with a significant reduction in MAP tissue burden when compared to control calves.3 Based on her results, Fecteau proposed that Ga compounds could be used as a potential feed additive (such as in milk replacers) in MAP-infected herds, and that their use during the period of high susceptibility and high exposure, when coupled with rigorous control measures, could reduce the incidence of JD in those herds.
 MAP’s effect on the microbiome: a possible key to prevention?
MAP’s effect on the microbiome: a possible key to prevention?
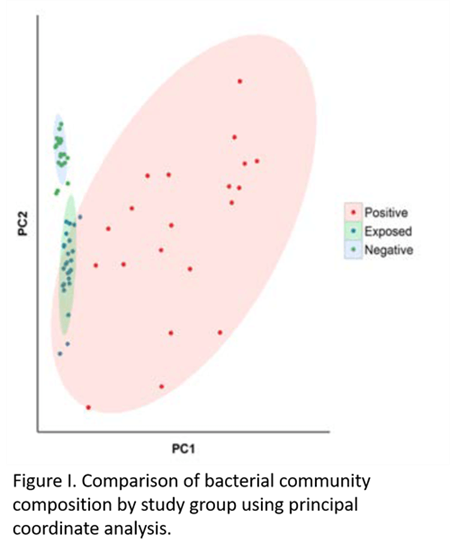
The gastrointestinal microbiome is fundamental to the overall health and production performance of most mammals. This diverse community of gastrointestinal bacteria exists in a delicate balance with the host. Disruption of this balance, termed “dysbiosis” can result in gastrointestinal dysfunction, including inflammation of the intestinal lining. Based on the inflammatory nature of JD, Dr. Fecteau next asked if MAP infection impacted the intestinal microbiome of infected cattle. With the help of her colleagues, Drs. Dipti Pitta and Raymond Sweeney from the Department of Clinical Studies-New Bolton Center, she investigated the diversity patterns of fecal bacterial populations in adult cattle infected with MAP, compared to those of uninfected-exposed cattle and uninfected-unexposed cattle using phylogenomic analysis. They demonstrated that fecal bacterial communities of MAP-positive cows varied significantly from those of cows from the exposed and negative groups (Figures 1 and 2).4 In collaboration with Dr. Daniel Beiting from the PennVet Center for Host Microbial Interactions, Fecteau investigated the impact of early MAP infection on the fecal bacterial populations of neonatal calves following experimental infection. Using a metagenomic analysis, they were able to conclude that the dysbiosis observed in MAP-infected calves (Figure 3) is a direct consequence of MAP infection, and that dysbiosis is likely to play a role in the intestinal inflammation observed in the affected animals. Notably, these findings may provide potential biomarkers for early detection and/or therapeutic probiotic agents designed to restore the microbiome to its proper balance, thereby reducing intestinal inflammation in MAP-infected animals.
One Medicine
Crohn’s disease (CD), an inflammatory gastrointestinal disease of humans, has many clinical and pathologic similarities to JD. The definitive cause of CD remains elusive and it is 3 Figure 1. Comparison of bacterial community composition by study group using principal coordinate analysis. Continued on page 4 PENN VET RESEARCH APRIL 2019 thought to result from a complex interaction of host susceptibility factors and an intense immune response to bacteria or other antigens in the intestines.5 Numerous studies investigating the role of MAP in CD have shown conclusively that MAP can be isolated from intestinal tissue of Crohn's patients (significantly more than controls), but the medical community still debates whether MAP causes the intestinal inflammation, or merely is able to colonize already-compromised intestinal tissues of afflicted individuals.6-7 In collaboration with Dr. Robert Baldassano and colleagues at the Children Hospital of Philadelphia, Drs. Fecteau, Sweeney and Beiting have turned their focus on improving MAP detection methods in pediatric CD patients to directly address this issue.
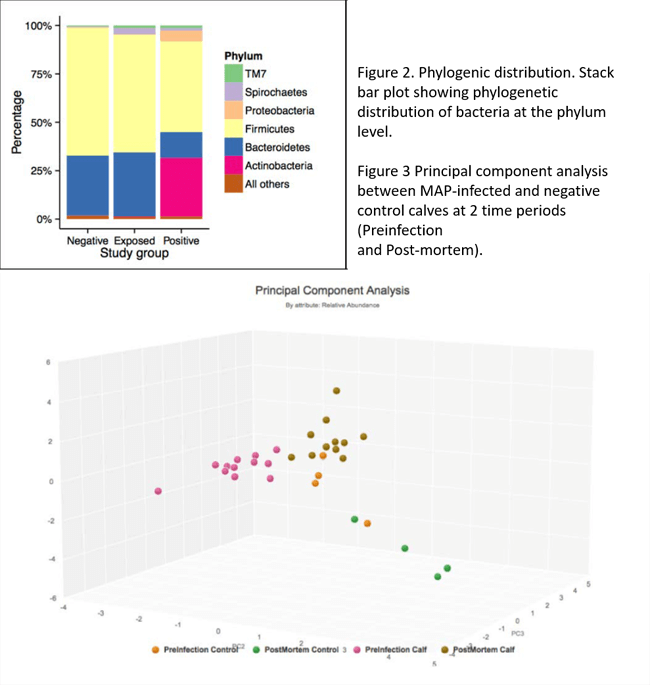
Figure 2. Phylogenic distribution. Stack bar plot showing phylogenetic distribution of bacteria at the phylum level.
Figure 3. Principal component analysis between MAP-infected and negative control calves at 2 time periods (Preinfection and Post-mortem).
Dr. Fecteau’s office is located in the New Bolton Center Hospital for Large Animals, Room 227.
References
- Fecteau ME, Fyock, TL, McAdams SC, Boston RC, Whitlock RH, Sweeney RW: Evaluation of the in vitro activity of gallium nitrate against Mycobacterium avium subsp. paratuberculosis. Am J Vet Res 2011; 72: 1243-1246.
- Monk CS, Sweeney RW, Bernstein LR, Fecteau ME. Serum and tissue concentration of gallium following oral administration of gallium nitrate and gallium maltolate in neonatal calves. Am J Vet Res 2016; 77: 151-155.78
- Fecteau ME, Whitlock RH, Fyock TL, McAdams SC, Boston RC, Sweeney RW: Antimicrobial activity of gallium nitrate against Mycobacterium avium subsp. paratuberculosis in neonatal calves. J Vet Intern Med 2011; 25: 1152-1155.
- Fecteau ME, Pitta DW, Vecchiarelli B, Indugu N, Kumar S, Gallagher SC, Fyock TL, Sweeney RW. Dysbiosis of the fecal microbiota in cattle infected with Mycobacterium avium subsp. paratuberculosis. PLOS ONE 2016; 11(8) e0160353.
- Marks DJ, Rahman FZ, Sewel GW, Segal AW. Crohn’s disease: an immune deficiency state. Clin Rev Alergy Immunol 2010; 3: 20-31.
- Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn’s disease using nucleic acid-base techniques: a systematic review and meta-analysis. Inflamm Bowel Dis 2008; 14: 401-410.
- Liverani E, Scaioli E, Cardamone C, Dal Monte P, Belluzzi A. Mycobacterium avium subspecies paratuberculosis in the etiology of Crohn’s disease, cause or epiphenomenon? World J Gastroenterol 2014; 20: 13060-13070.