
Dr. Kyla Ortved is an assistant professor of Large Animal Surgery at New Bolton Center, the large animal campus of the University of Pennsylvania School of Veterinary Medicine, located in Kennett Square, Pennsylvania. She received her DVM degree from the University of Guelph in 2006 and completed her large animal surgical residency at Cornell University in 2010. Dr. Ortved became boarded with the American College of Veterinary Surgeons in 2011. Following her residency, Dr. Ortved went on to obtain a PhD in equine cartilage repair at Cornell University in June 2014 and joined the faculty at Cornell Ruffian Equine Specialists in July 2015 as Clinical Assistant Professor of Equine Surgery. In January 2016, Dr. Ortved became boarded with the American College of Sports Medicine and Rehabilitation. She joined the faculty at New Bolton Center in February 2016 as a large animal orthopedic surgeon. Dr. Ortved’s research program at New Bolton Center is focused on joint disease, using the horse as a model for human disease. Specifically, she is interested in developing cell and gene therapies to improve cartilage repair and prevent the development of osteoarthritis.
Developing new therapies to prevent post traumatic osteoarthritis (PTOA) in the horse and the rider
Joint injuries are overwhelmingly common in both human and equine athletes. Chondrocytes, the sole cell type in cartilage, are responsible for producing and maintaining the extra-cellular matrix (ECM), which affords remarkable tensile and compressive strength to the joint surface. The intrinsic healing capabilities of cartilage are poor, in part due to its avascular and hypoxic nature[1]. Therefore, posttraumatic osteoarthritis (PTOA) commonly develops following sufficient joint trauma, whether sustained during an acute injury or accumulated overtime. Unfortunately, PTOA is a progressive, debilitating disease that currently lacks any effective treatment. Pain and decreased mobility are addressed with systemic non-steroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroids, rest and rehabilitation. The end stage therapy for OA joints in humans is total joint replacement, while select joints in the horse can be treated with surgical arthrodesis. Both of these procedures are invasive and expensive interventions. Dr. Ortved’s research program is focused on understanding the pathophysiology of PTOA and developing gene and cell-based therapies to help regenerate cartilage and prevent the development of PTOA following joint injury. Due to the many similarities in joint biomechanics and propensity for PTOA, the horse serves as an excellent large animal model for human joint disease.
Cell-based therapies aim to return damaged or injured tissue to a more normal structure and function. An overarching goal in cellbased repair is to restore the articular surface, thereby preventing further joint degradation. Although bone marrow-derived MSCs (BM-MSCs) have been the most frequently used MSC type for cartilage repair to date, full chondrogenic differentiation has been disappointing. Therefore, Dr. Ortved is currently investigating the chondrogenic differentiation capability of synovial membrane-derived mesenchymal stem cells (SD-MSCs). SDMSCs may have superior chondrogenesis due to a common progenitor cell between synovium and cartilage[2]. Recent experiments using flow cytometry in the Ortved laboratory have demonstrated a similar immunophenotype between BM-MSCs and SD-MSCs, with SD-MSCs expressing the appropriate markers of stemness including CD29, CD44, CD90, CD105 and MHCI and lacking expression of exclusion markers including CD45, CD79, and MCHII (Figure 1) [3]. They have also shown that SD-MSCs have increased proliferative capacity in vitro, making timely culture expansion of these cells achievable. Trilineage differentiation assays are being performed to assess chondrogenic differentiation, with the ultimate goal of defining a source of adult MSCs suitable for resurfacing cartilage lesions to facilitate healing.

Figure 1. Flow cytometric analysis of BM-MSCs (top panel) and SD-MSCs (bottom panel). Green lines represent negative isotype control staining and purple lines represent cell surface marker staining. The percentage of positive cells is in the upper right-hand corner of the histograms. Analysis showed that both types of MSCs have similar immunophenotypes consistent with equine stemness.
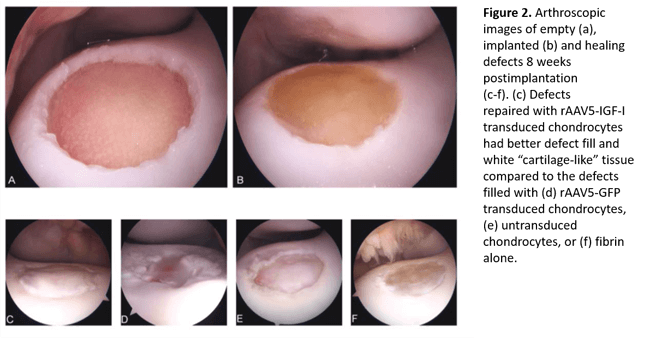
Gene therapy also has the potential to bolster the weak healing response in articular cartilage. Previous work by Dr. Ortved has demonstrated improved cartilage repair in large, full-thickness chondral defects created in the lateral trochlear ridge of the equine femur using autologous chondrocytes transduced ex vivo with an adeno-associated virus (AAV) vector overexpressing the anabolic protein IGF-I[4] (Figure 2). Dr. Ortved has also demonstrated that AAV vectors can be used to safely transduce equine articular cells in vivo with efficient, sustained expression of a therapeutic transgene[5]. More recently, Dr. Ortved has been investigating AAV-mediated overexpression of interleukin-10 (IL-10), an immunomodulatory cytokine. She showed that L-10 overexpression downregulates expression of proinflammatory mediators in inflamed chondrocytes in vitro. Next, Dr. Ortved is planning on assessing the protective effects of this therapy in vivo to determine if it can mitigate the post-traumatic inflammatory response that occurs following joint injury.
Despite the robust nature of healthy cartilage, the meager healing response that follows trauma leads to serious health consequences long-term. Indeed, PTOA is often career ending in both equine and human athletes, and is one of the most common disabilities of the aging human population. Early studies in the Ortved laboratory have identified promising cell and gene therapeutic strategies that may improve repair of cartilage lesions and modulate the posttraumatic inflammatory response in the joint. As the horse is an invaluable large animal model for human joint disease, Dr. Ortved will continue to investigate joint therapies that will hopefully allow both horse and rider to ride off into the sunset a bit more comfortably.
Dr. Ortved’s research has been funded by NIH, Grayson-Jockey Club Research Foundation, Harry M. Zweig Memorial Fund for Equine Research, and the Raymond Firestone Trust. Dr. Ortved’s laboratory is located Myrin 104, New Bolton Center.
References
- Poole, A.R., Kojima, T., Yasuda, T., Mwale, F., Kobayashi, M. and Laverty, S. (2001) Composition and structure of articular cartilage: a template for tissue repair. Clin. Orthop. Relat. Res. 391 Suppl, S26-33.
- Archer, C.W., Dowthwaite, G.P. and Francis-West, P. (2003) Development of synovial joints. Birth Defects Res C.EmbryoToday. 69, 144–155
- Fülber, J., Maria, D.A., Silva, L.C.L.C. da, Massoco, C.O., Agreste, F. and Baccarin, R.Y.A. (2016) Comparative study of equine mesenchymal stem cells from healthy and injured synovial tissues: an in vitro assessment. Stem Cel Res. Ther. 7, 35.
- Ortved, K.F., Begum, L., Mohammed, H.O. and Nixon, A.J. (2015) Implantation of rAAV5-IGF-I Transduced Autologous Chondrocytes Improves Cartilage Repair in Full-thickness Defects in the Equine Model. Mol. Ther. 23, 363–373.
- Ortved, K., Wagner, B., Calcedo, R., Wilson, J., Schaefer, D. and Nixon, A. (2015) Humoral and cellmediated immune response, and growth factor synthesis after direct intraarticular injection of rAAV2-IGF-I and rAAV5-IGF-I in the equine middle carpal joint. Hum. Gene Ther. 26, 161–71.