Ciliopathies are diseases that affect the cilia, sensory organelles that most mammalian cells possess and which play a critical role in many biological functions. One such disease is Senior Løken Syndrome, a rare condition that can involve both a severe kidney disease and the blinding disease Leber congenital amaurosis, or LCA.
A decade ago, researchers from the University of Pennsylvania and colleagues identified a dog with a similar blinding condition, and in 2013 they reported the causative gene.
Now the Penn scientists report that they’ve directly compared the disease course between humans and dogs and found remarkable similarities.
Given the crucial role that animal models play in pushing disease therapies forward, the researchers are optimistic about developing therapies that treat the condition first in dogs, and eventually in people.
“When we started characterizing the disease, we found striking similarities between a subset of human patients with LCA and dogs with this mutation,” said Gustavo D. Aguirre, a co-senior author and professor of medical genetics and ophthalmology at Penn’s School of Veterinary Medicine. “We’re very enthused about the potential to narrow down a therapeutic window for this disease and begin testing translational therapies.”

The research is published in the journal Human Molecular Genetics and included a cross-disciplinary team from Penn. In addition to Aguirre, Penn Vet contributors included co-senior author William A. Beltran, co-first authors Louise M. Downs and Erin M. Scott, Simone Iwabe, Valerie Dufour, Kristin L. Gardiner, Sem Genini and Luis Felipe Marinho. As part of a long-standing collaboration with the Perelman School of Medicine’s Scheie Eye Institute, Aguirre and Beltran’s team partnered with Artur V. Cideciyan and Samuel G. Jacobson as well as Alexander Sumaroka, Mychajlo S. Kosyk and Malgorzata Swider. Geoffrey K. Aguirre of the Perelman School of Medicine rounded out the research team, contributing his expertise in neurology, as neurological factors can present in the human form of the syndrome.
When the Penn team helped discover the ciliopathy in dogs, they noticed something unusual.
“What is striking about the canine disease is that although the dogs lack functional vision in daylight,” said Aguirre, “the cone cells, which are the photoreceptor cells that function in daylight, are still there.”
“They’re there but they are structurally very compromised,” added Beltran, an associate professor of ophthalmology at Penn Vet. “The key question to be answered now is whether therapeutic intervention will not only halt the degenerative process but repair these abnormal cones and restore day vision. We have exciting preliminary gene therapy results that seem to confirm that this is indeed possible.”
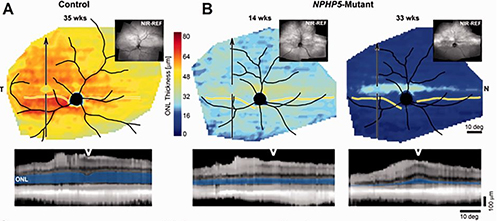
In a 2011 study, the Vet researchers’ Scheie Institute colleagues, Cideciyan and Jacobson, both professors of ophthalmology at Penn Medicine, had observed something similar in some of their human patients with LCA. Their central retina contained cone cells that were preserved but lacked function. The rod photoreceptors, responsible for dim light vision, deteriorated early in life.
In these people and in the dogs with the ciliopathy, the same gene was involved: NPHP5. To find out how the genetic mutation was affecting cone cell function and rod deterioration, the researchers turned to the canine model.
Erin Scott, at the time a veterinary student who was a research Merck/Merial scholar in Beltran’s lab, and Louise Downs, a postdoctoral researcher in Aguirre’s lab, tracked the disease in dogs, finding similar patterns as in humans. The cone cells remained present, though non-functional and structurally altered, until very late in disease, around 42 weeks of age, when they progressively degenerated. They observed that fairly early in life, around six weeks, the majority of cone cells in the affected dogs had failed to form the outer segment, which is connected by the cilia to the inner segment, and is the light capturing structure of the photoreceptors. The inner segment, too, appeared disordered, similar to what had been reported in human patients.
The researchers examined the expression of 112 genes in the retina that are known to play a direct role in vision, or that are involved in cell death or survival to see if and how NPHP5 mutation influenced them. They found in NPHP5 mutant dogs there were changes in expression in 33 of these genes some of which also altered in two other canine forms of retinal degeneration.
Finally, the researchers assessed whether, as in humans with mutations in NPHP5, kidney function or structure was affected, but found that dogs did not appear to be affected by these other features of the ciliopathic syndrome.
Research to deliver via gene therapy a normal copy of the NPHP5 gene in the canine model of disease is already underway, with promising early results.
The study was supported by the National Institutes of Health, Foundation Fighting Blindness, NIH-Merck/Merial Summer Research Fellowship, Hope for Vision, Macula Vision Research Foundation, Alcon Research Institute and Research to Prevent Blindness.