Bellwether Magazine
Discover Bellwether, Penn Vet’s premier biannual publication. Explore exciting stories and insights, and don’t miss the chance to delve into our extensive Bellwether Archives!
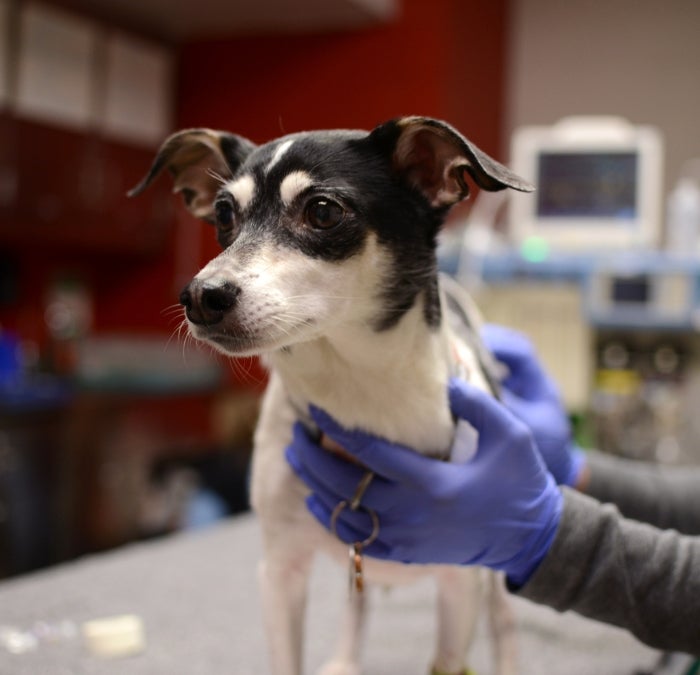
Featured Article
One Tiny Dog’s Outsized Contribution to Brain Surgery
Geddy Lee has lived a big life for a little dog.
Featured Articles

Penn Vet Holds Ribbon Cutting for New $2.8 Million Richard Lichter Advanced Dentistry and Oral Surgery Suite
The University of Pennsylvania School of Veterinary Medicine (Penn Vet) commemorated the official re-opening of the newly named Richard Lichter Advanced Dentistry and Oral Surgery Suite at Ryan Hospital with…

From Bird Fractures to Human Shoulders
As a child in Central Pennsylvania, Stephen Peoples, V’84, loved science and the natural world.

In the Office with Igor Brodsky, PhD
In his Philadelphia-based lab, Igor Brodsky is on a mission to understand how the body recognizes and responds to invading organisms that cause several diseases.





