Featured

Penn Vet News
Featured News

Penn Vet Welcomes New Director of Alumni Relations to Strengthen Lifelong Connections and Advance School Mission
The School of Veterinary Medicine at the University of Pennsylvania (Penn Vet) has announced the appointment of Laura Brown as the new director of alumni relations. With a strong background…

Penn breaks ground on Gail P. Riepe Center for Advanced Veterinary Education (link is external)
On a sunny, 71° day in Kennett Square, the School of Veterinary Medicine and broader University of Pennsylvania community celebrated the milestone groundbreaking of the Gail P. Riepe Center for…


Events at Penn Vet
Explore one of the many events we hold that help us positively impact our community and the greater Philadelphia region.

In Principle and Practice
Highlights

Penn Vet Welcomes New Director of Alumni Relations to Strengthen Lifelong Connections and Advance School Mission
The School of Veterinary Medicine at the University of Pennsylvania (Penn Vet) has announced the appointment of Laura Brown as the new director of alumni relations. With a strong background…

Penn breaks ground on Gail P. Riepe Center for Advanced Veterinary Education (link is external)
On a sunny, 71° day in Kennett Square, the School of Veterinary Medicine and broader University of Pennsylvania community celebrated the milestone groundbreaking of the Gail P. Riepe Center for…
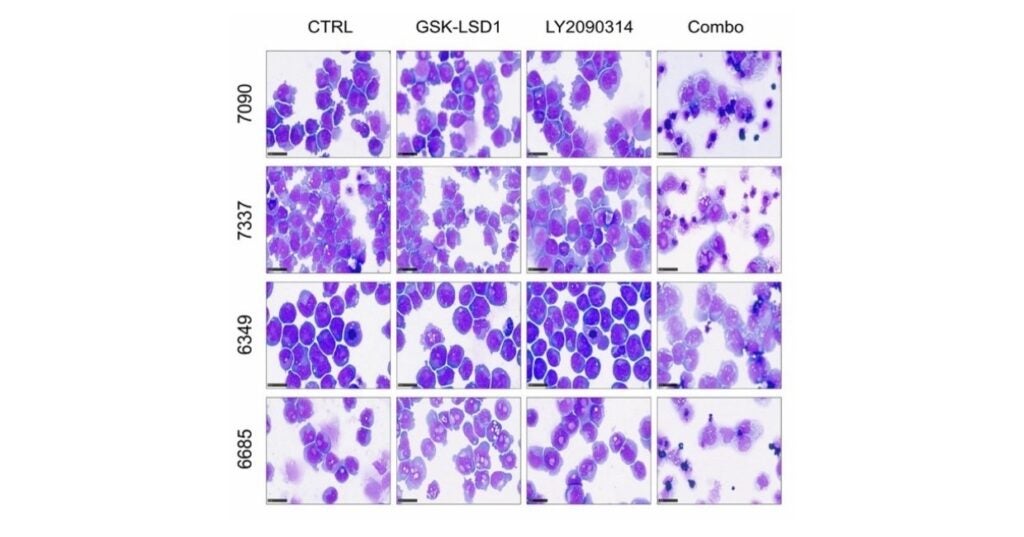
New Study Identifies Promising Inhibitor Combination for Hard-to-Treat Leukemia Subtypes
Faculty In This Story Assistant Professor of Biomedical Sciences M. Andrés Blanco, PhD, from the University of Pennsylvania’s School of Veterinary Medicine (Penn Vet) and investigators from the Universities of…
Achievements

New Study Identifies Promising Inhibitor Combination for Hard-to-Treat Leukemia Subtypes
Faculty In This Story Assistant Professor of Biomedical Sciences M. Andrés Blanco, PhD, from the University of Pennsylvania’s School of Veterinary Medicine (Penn Vet) and investigators from the Universities of…

New genetic cause of blindness in dogs (link is external)
In collaboration with a foundation that breeds service dogs for the visually impaired, researchers at the School of Veterinary Medicine at the University of Pennsylvania and the University of Padova…

University of Pennsylvania Avian Influenza Experts Provide Briefing to Pennsylvania Policy Makers
As concerns about the highly pathogenic avian influenza (HPAI) outbreak continue to grow, experts from the University’s School of Veterinary Medicine (Penn Vet) and Perelman School of Medicine (PSOM) visited…
In the Media
Bellwether Features
Ryan Hospital Staff Veterinarians Talk Life in Emergency Services and Critical Care
Drs. Catalina Montealegre and Charles Garneau-So participate in a Q&A.

Ryan Hospital Launches Canine Hydrotherapy Services
Addition of Underwater Treadmill Expands Treatment Options for Arthritis, Rehabilitation

Tara Gaab, V’17
From Classroom to Farms to Back Again

A Family Affair
Robert Marookian supports New Bolton Center in honor of his mother and brother

In the Office with Dr. Deborah Silverstein
Get to know Professor of Emergency & Critical Care Deborah Silverstein.
For the media
Press Inquiry
For all press inquiries, please the form below. This form is for media purposes only. If you are an animal owner, contact Ryan Veterinary Hospital for Companion Animals or, for our large animal hospital, New Bolton Center.
Non-News, Commerce-Affiliated Content Site Inquiries
The Office of Communications is selective in responding to requests from freelance writers on assignment for non-news, content building sites.
- All interviews with clinicians, faculty, staff and/or students in association with Penn Vet, Ryan Hospital, or New Bolton Center must be arranged through and facilitated by a representative from the Office of Communications. Please do not contact such individuals directly.
- When submitting a media or interview inquiry, please fill out this form. Include your name, contact information, and general details of your pitch including important deadlines.
- Visits to Penn Vet’s campuses and/or hospitals by members of the media must be arranged directly through a representative from the Office of Communications. For the well-being and privacy of our animal patients and their owners, a representative from the Office of Communications must also accompany any arranged visits.
- If an interview requires a Penn Vet clinician, faculty or staff member, or a student to be recorded on video, please be aware that a signed copy of the University’s video authorization form must be submitted to and confirmed by a representative from the Office of Communications prior to any taping. Additionally, an an Appearance Release must be supplied to the Office of Communications for review and approval prior to obtaining a signed agreement from the individual that is to appear on camera.





