Hope for cell-based retinal repair: Penn Vet study finds key to improving survival of transplanted cells
Researchers identify metabolic stress as an obstacle to photoreceptor transplantation highlighting a critical window for therapeutic intervention
Cell replacement therapy offers new hope for millions of people affected by retinal degenerations (RDs) – a group of blinding conditions caused by the loss of light-sensing photoreceptor cells in the retina. Among the most promising approaches is the transplantation of stem cell-derived partially differentiated photoreceptor cells, known as precursor cells, to replace those lost to disease. But a persistent hurdle remains: many of the transplanted cells do not survive long enough to integrate or restore vision.
Now, researchers from the Division of Experimental Retinal Therapies (ExpeRTs) at the University of Pennsylvania School of Veterinary Medicine (Penn Vet), led by Raghavi Sudharsan, PhD, and William A. Beltran, DVM, PhD, DECVO, in collaboration with David Gamm, MD, PhD, at the University of Wisconsin-Madison and Petr Baranov, PhD, at Harvard Medical School, have uncovered a key reason for this early transplant failure. In a newly published study in Stem Cell Research & Therapy, the team reports that photoreceptor precursor cells experience widespread death within the first few days after being injected into the subretinal space, even under conditions of effective immune suppression. The culprit, they found, is acute metabolic stress triggered by a sudden shift from a nutrient-rich culture environment into the relatively nutrient-deprived conditions of the subretinal space – a transition that triggers rapid cell loss during the first few days after transplantation. The study points to a critical need for strategies that help donor cells survive this abrupt metabolic transition and better adapt to the challenging conditions of the host retina.
“These findings really emphasize how vulnerable transplanted cells are during those first few days,” said Sudharsan, an Assistant Professor of Experimental Ophthalmology. “We’ve long focused on immune rejection as the main threat, but this shows that metabolic stress is an equally critical challenge – one that demands new strategies if we want these therapies to succeed.”
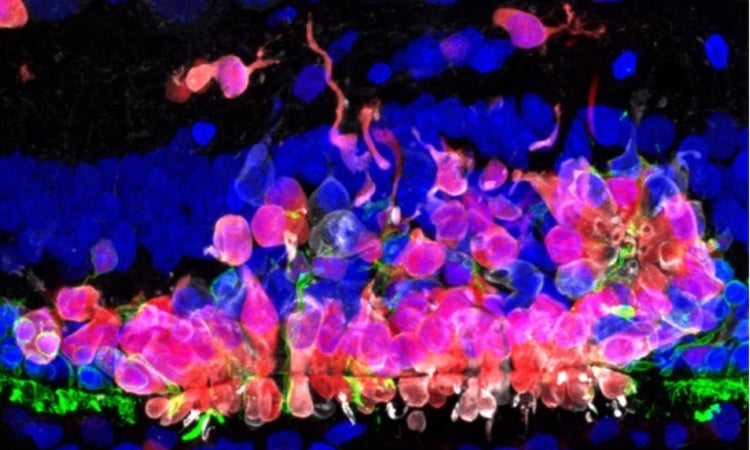
Using non-invasive imaging, the researchers found that early donor cell death was consistently observed, regardless of whether the host retina was healthy or already undergoing degeneration. To uncover the underlying cause, they performed single-cell RNA sequencing on transplanted cells in healthy retinas, which revealed signatures of acute metabolic stress. These findings were further validated by immunohistochemistry, confirming that the transplanted cells were undergoing oxidative damage and cell death shortly after transplantation.
Still, the study revealed a positive aspect: a subset of donor cells did survive and continued to mature after transplantation. In retinas that retained some of the native photoreceptors, these surviving cells were even able to begin forming structures that resembled synaptic connections. The timing of transplantation proved to be crucial. In models where portions of the retina’s photoreceptor layer were still intact, donor cells could integrate into the host tissue. But in cases of end-stage degeneration, where the retinal architecture was too far gone, transplanted cells failed to survive.
“These findings suggest that some damage to the native photoreceptor layer is actually necessary to allow transplanted cells to integrate,” said Beltran, the Corinne R. and Henry Bower Professor of Ophthalmology and director of the Division of ExpeRTs. “But if degeneration progresses too far, the retinal environment becomes too hostile to support their survival.”
The study highlights a critical therapeutic window: a stage in disease progression where the retina is damaged enough to permit integration, but not so far gone that it can no longer support transplanted cells.
Supported by the National Eye Institute and several vision research foundations, the study defines a major challenge in retinal cell therapy and points to tangible strategies for overcoming it. Therapies that aim to replace lost photoreceptors must not only be timed appropriately but also include strategies to protect donor cells from early metabolic stress – such as preconditioning, scaffold-based delivery, or nutrient support.
“As the field moves closer to clinical translation,” said Sudharsan, “understanding how both the transplanted cells and the host retina respond will be essential to designing therapies that actually succeed in patients.”
Sudharsan’s and Beltran’s coauthors on the work were Penn Vet’s Natalia Dolgova, Jennifer Kwok, Alexa Gray, Yu Sato, John H. Wolfe, and Gustavo D. Aguirre; the University of Wisconsin-Madison’s Agustin Luz Madrigal, Praveen Joseph Susaimanickam, and David M. Gamm; and the Harvard University’s Emil Kriukov, and Petr Baranov.
Raghavi Sudharsan is an Assistant Professor of Experimental Ophthalmology and member of the Division of Experimental Retinal Therapies in the Department of Clinical Sciences & Advanced Medicine at the School of Veterinary Medicine, University of Pennsylvania.
Petr Baranov is an Assistant Professor of Ophthalmology and Associate Director of the Ocular Regenerative Medicine Institute, in the Department of Ophthalmology at Harvard Medical School.
John H Wolfe is a Professor of Pathology in the Department of Pathobiology at the School of Veterinary Medicine, University of Pennsylvania and a Stokes Investigator of the Joseph Stokes, Jr. Research Institute, Children’s Hospital of Philadelphia
Gustavo D. Aguirre is a Professor of Medical Genetics and Ophthalmology and member of the Division of Experimental Retinal Therapies in the Department of Clinical Sciences & Advanced Medicine at the School of Veterinary Medicine, University of Pennsylvania.
David M Gamm is a Professor of Ophthalmology and Visual Sciences, and the RRF Emmett A. Humble Distinguished Director McPherson Eye Research Institute at the University of Wisconsin-Madison.
William A. Beltran is the Corinne R. and Henry Bower Endowed Professor of Ophthalmology and director of the Division of Experimental Retinal Therapies in the Department of Clinical Sciences & Advanced Medicine at the School of Veterinary Medicine, University of Pennsylvania.
This study was supported by the National Eye Institute (grants U24-EY029890, RO1-EY006855, and P30-EY001583), Fighting Blindness Canada Vision 20/20, The Foundation Fighting Blindness, The Van Sloun Fund for Canine Genetic Research, Research to Prevent Blindness, the Sandra Lemke Trout Chair in Eye Research, and the Retina Research Foundation.
The study involved a partnership with a number of institutions and labs.

Related News

Behind the Breakthroughs: Amy Johnson
Balancing clinical care with scientific inquiry, Penn Vet’s Amy Johnson leads efforts to decode the complexities of neurologic diseases in horses

Penn Vet’s Wildlife Futures Seek to Unravel the Mystery of the Disappearing Barn Owl
Penn Vet’s Wildlife Futures Program and the Pennsylvania Game Commission (PGC) have been engaged in a collaborative effort to identify the causes of these owls’ decline and any actions that…

Penn Vet’s Annual Research Retreat Calls for the Power of Synergy in Challenging Times
Noting the challenges ahead, but celebrating the many breakthroughs at hand, the University of Pennsylvania School of Veterinary Medicine (Penn Vet) community gathered for their 31st annual Research Retreat held…
About Penn Vet
Ranked among the top ten veterinary schools worldwide, the University of Pennsylvania School of Veterinary Medicine (Penn Vet) is a global leader in veterinary education, research, and clinical care. Founded in 1884, Penn Vet is the first veterinary school developed in association with a medical school. The school is a proud member of the One Health initiative, linking human, animal, and environmental health.
Penn Vet serves a diverse population of animals at its two campuses, which include extensive diagnostic and research laboratories. Ryan Hospital in Philadelphia provides care for dogs, cats, and other domestic/companion animals, handling more than 30,000 patient visits a year. New Bolton Center, Penn Vet’s large-animal hospital on nearly 700 acres in rural Kennett Square, PA, cares for horses and livestock/farm animals. The hospital handles more than 6,300 patient visits a year, while our Field Services have gone out on more than 5,500 farm service calls, treating some 22,400 patients at local farms. In addition, New Bolton Center’s campus includes a swine center, working dairy, and poultry unit that provide valuable research for the agriculture industry.
