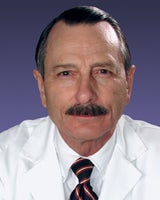
Aging, Metabolism, and Mitochondria
Research in the Department of Biomedical Sciences on aging and metabolism encompasses broad aspects of chemical, biological and oxidative stress as well as neuronal stress. Our labs have produced groundbreaking discoveries in the study of spermatogonial stem cells and its niche, transgenerational germ cell reprogramming and the role of transcription factor NF-kB in chronic inflammatory diseases.
These studies employ diverse cutting edge approaches including genetic models of human disease, metabolic profiling, microRNA analysis, metabolic activation of environmental toxins, and elucidation of an array of signaling pathways.
Research from our labs contributes to efforts to better understand and develop treatments for age related diseases including osteoporosis and related muscloskeletal disorders, cancer, reproductive diseases, and maternal stress on development.
Contact the Biomedical Sciences Department
Our department is supported by a core of dedicated professional and administrative staff.
Department of Biomedical Sciences
216E Old Vet Quad
3800 Spruce Street
Philadelphia, PA 19104