Research
Stem Cell Therapy
Cell-based therapies aim to return damaged or injured tissue to a more normal structure and function. An overarching goal in stem cell therapy is to protect and restore cartilage, thereby preventing further joint degradation. We are harnessing the chondrogenic differentiation capabilities as well as the immunomodulatory properties of stem cells to address cartilage injury and global joint inflammation the ensues following trauma. By mitigating joint inflammation, we are hoping to prevent inflammation-driven degeneration of the joint.
With FDA approval, our lab also offers a bone marrow-derived mesenchymal stem cell (MSC) culture service for horses. This innovative treatment has shown efficacy in the treatment of tendon and ligament injuries, osteoarthritis, and meniscal injuries. Our lab cultures stem cells in house for treatment of horses at New Bolton or on the farm by a veterinarian. For more information, please contact Kyla Ortved, DVM, PhD at kortved@vet.upenn.edu and Renata Linardi, DVM, PhD at rlinardi@vet.upenn.edu.
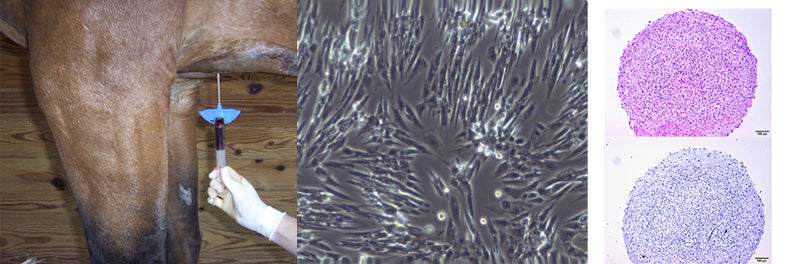
Bone marrow is harvested from the sternum of a sedated horse and culture-expanded in the lab. We are using these cells to form cartilage-like pellets through chondrogenic differentiation, in order to optimize a cell-source that could be used to repair cartilage defects in the joint.
Extracellular Vesicles
Extracellular vesicles (EV) carry important signaling proteins, lipids and RNA. These membrane bound particles are released by cells, including stem cells, and can be used as a sensitive biomarker of disease and a potential therapeutic due to their broad and optimizable immunomodulatory capabilities. We are investigating EVs in joint fluid of horses with naturally-occurring PTOA and EVs collected from stem cells cultured in different environments.
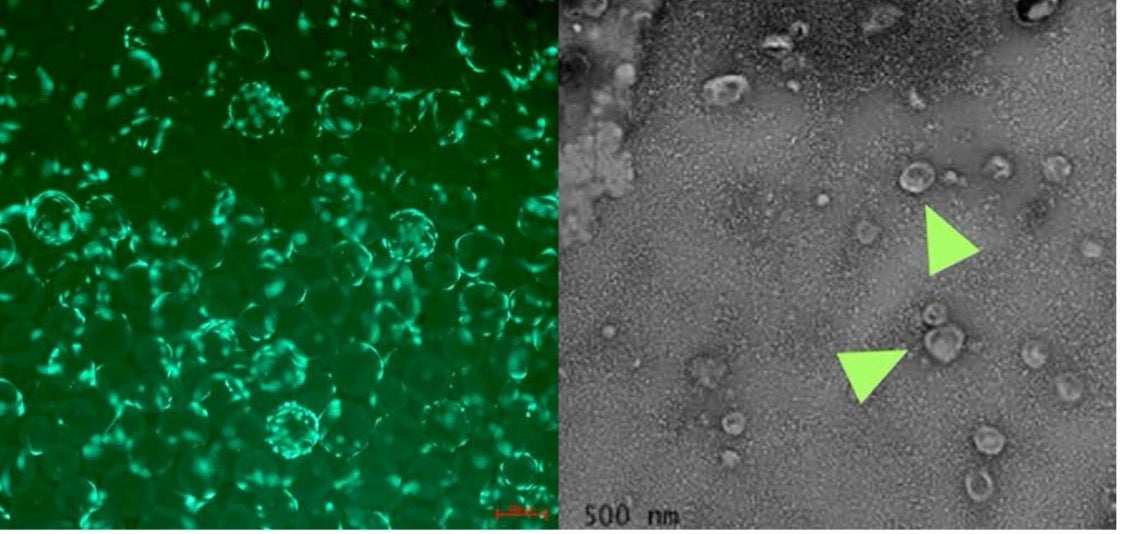
Mesenchymal stem cells (fluorescent green) cultured on microcarrier beads in order to optimize EV production (left). Transmission electron microscopy (TEM) image of EVs derived from joint fluid (right).
Gene Therapy
Gene therapy has the potential to bolster the weak healing response in cartilage and decrease joint inflammation. Dr. Ortved has demonstrated improved cartilage repair in large, full-thickness chondral defects created in the lateral trochlear ridge of the horse femur using autologous chondrocytes transduced ex vivo with an adeno-associated virus (AAV) vector overexpressing the anabolic protein IGF-I. We have also demonstrated the ability of the anti-inflammatory cytokine IL-10 to protect cartilage from degeneration and have shown that IL-10 can be safely overexpressed in vivo following direct injection of AAV-IL-10 into horse joints. We are currently assessing the effects of IL-10 gene therapy in a clinical trial to determine if it can mitigate the joint inflammation and improve comfort.

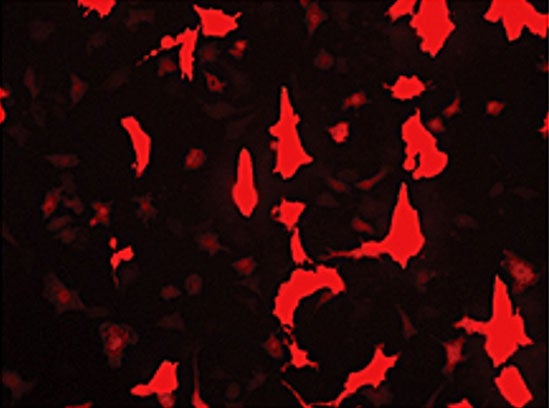
Horse chondrocytes have been transduced with an AAV vector, expressing red fluorescent protein.
Immunomodulation in PTOA
Since PTOA is driven by inflammation, we are investigating multiple ways to mitigate the inflammatory cascade and improve the articular environment. Our lab is focused on characterizing and optimizing cytokines, growth factors, and extracellular vesicles in autologous hemoderivatives for direct intraarticular therapy. Hemoderivatives are processed from the patient’s blood for direct or local injection of the final product and help to direct optimal healing of injured tissue.
