Brinster Laboratory of Reproductive Physiology
Our Research
Our research has involved studies on mammalian germ cells and early embryos. Initially, we developed a culture system and manipulation techniques for mouse eggs that are the foundation for subsequent mammalian egg and embryo experiments in the field, including nuclear transfer and in vitro fertilization of human eggs.

Publications
July 2024: Dr. Brinster has 411 total publications, approximately 322 are in peer-reviewed journals and 89 are chapters/reviews. Included in the publications are: 30 in Nature, 18 in Cell, 13 in Science, and 35 in Proc. Natl. Acad. Sci.
His research contribution/impact is ranked 1st of 173,000 contributors in Veterinary Medicine in the world and 8th of one million contributors in Agriculture and Natural Resources in the world in Scholars GPS. with an h-index = 153
He is ranked #128 in the world and #96 in the United States among top scientists for 2024 in Research.com with a D-index (Discipline h-index) = 152.
In: Fertility Preservation: Principles & Practice, Second Edition. Chap. 18, Fertility Preservation Strategies in the Male. Ginsberg, J. and Brinster, R.L. Transplantation of Cryopreserved Spermatogonia. Editors: Donnez, J. and Kim, S., Cambridge University Press. 2021. ISBN: 9781108494595.
FGF9 promotes mouse spermatogonial stem cell proliferation mediated by p38 MAPK signaling. Yang, F., Whelan, E., Guan, X., Bingquan, D., Shu, W., Sun, J., Avarbock, M.R, Wu, X., Brinster, R.L. Cell Prolif. 2021;54: e12933. https://doi.org/10.1111/cpr.12933.
Roles of Stra8 and Tcerg1l in retinoic acid induced spermatogonial differentiation in mouse. Sinha N, Whelan EC, Tobias JW, Avarbock M, Stefanovski D, Brinster RL. Biology of Reproduction, 2021, 1-16 doi:10.1093/biolre/ioab093
Spermatogenesis after more than 20 years of cryopreservation of rat spermatogonial stem cells reveals an important impact in differentiation capacity. Whelan EC, Yang F, Avarbock MR, Sullivan MC, Beiting DP, Brinster RL (2022) PLOS Biol 20(5): e3001618. https://doi.org/10.1371/journal. pbio.300.
Director, Brinster Laboratory
Ralph Brinster, VMD, PhD
Professor of Physiology
Find Us
University of Pennsylvania
School of Veterinary Medicine
3800 Spruce Street
Philadelphia, PA 19104-4539
Our Team

Mary Avarbock

Clarence Freeman

Rose Naroznowski

Carolyn Pope
Many colleagues and staff have supported this lab through their years of service: Mary Avarbock, 56 years; Clarence Freeman, 33 years; Rose Naroznowski, 39 years; and Carolyn Pope, 40 years. Together, they have dedicated a total of 168 years. Our research would not have been possible without their support and collaboration.

A new study from Penn Vet on germ cell gene regulatory networks paves way to determine cause of cryopreservation fertility failures
A study published in Stem Cell Reports, led by Eoin Whelan, PhD, and Ralph Brinster, VMD, PhD, and a team of researchers at the University of Pennsylvania’s School of Veterinary…
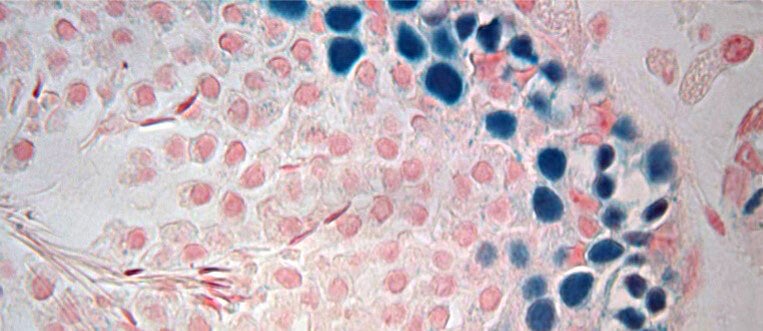
Frozen testicular tissue still viable after 20 years (link is external)
A new study in rats has shown that male testis tissue that is cryopreserved can be reimplanted after more than 20 years and will go on to make viable sperm.
