Hunter Laboratory
About Us
For nearly 30 years, the Hunter laboratory has focused on understanding how the immune response to Toxoplasma gondii is regulated to allow the development of protective immunity while limiting T cell-mediated pathology in multiple sites, including the gut and brain.
Our Research
Christopher Hunter has been working on various aspects of basic parasitology since 1984, and for the last 25 years, has been focused on understanding how the protective immune response to Toxoplasma gondii develops and how this relates to other parasitic infections.
The Hunter laboratory has focused on the innate events that lead to the development of long-term protective immunity mediated by T and B cells. These studies led us to develop expertise in cytokine biology, and while the focus has been on understanding their role in infectious disease, these findings are frequently relevant to cytokine function in autoimmunity and inflammatory processes associated with human disease.
For example, as part of studies to understand how IL-12 family members affect immunity to T. gondii, we showed that IL-27 is important in limiting the T cell-mediated infection-induced inflammation. We have defined the mechanisms used by IL-27 to influence the immune system, and our work has been shown to be relevant to inflammatory processes in multiple experimental systems that include other infections as well as models of auto-immune inflammation, asthma, and cancer.
Since toxoplasma causes a chronic infection in the brain, we also have a long-term interest in the neuropathogenesis of infectious diseases and how lymphocytes access and operate in this immune-privileged site.
We routinely analyze multiple innate immune parameters and quantify DC, macrophage, NK, T, and B cell responses to infection. We also utilize different combinations of transgenic parasites and TCR transgenic T cells to provide higher resolution analysis of individual parasite-specific CD4 and CD8 T cell populations and apply multi-photon microscopy to image the innate and adaptive response to T. gondii.
As part of this research program, Dr. Hunter has supervised more than 50 students from multiple programs, including Penn undergraduates, VMD summer students, graduate students from the MVP and Immunology graduate groups, combined degree students (VMD/PhD and MD/PhD), and PhD candidates and undergraduates from the UK. He has been responsible for the training of 15 post-docs and 29 students (23 PhD, two Master’s, and four in training). Almost all of these individuals are still in science, with trainees in faculty positions, industry, and science policy.

An IL-12 mRNA-LNP Adjuvant Enhances mRNA Vaccine-Induced CD8 T Cell Responses, Aunins EA, et al. Science Immunology (2025), 10(108).

Image stream analysis of STAT6 activation in cells from infected mice. Zsgreen1+ macrophages that contain a single intracellular parasite (top row) or are uninfected (lower row) are shown. Cells were stained with DAPI to identify the host cell nucleus as well as with a p-STAT6 antibody. The bright field overlay shows that in both cells, the pSTAT6 is localized in the host nucleus.
Publications
An IL-12 mRNA-LNP Adjuvant Enhances mRNA Vaccine-Induced CD8 T Cell Responses
Aunins EA, Phan AT, Alameh MG, Dwivedi G, Cruz-Morales E, Christian DA, Tam Y, Bunkofske ME, Peñafiel AZ, O’Dea KM, Merolle M, Furey C, Scott P, Vonderheide RH, Hensley SE, Kedl RM, Weissman D, Hunter CA. Science Immunology (2025), 10(108). PMID 40478935.
Immune targeting and host-protective effects of the latent stage of Toxoplasma gondii. Eberhard JN, Shallberg LA, Winn A, Chandrasekaran S, Giuliano CJ, Merritt EF, Willis E, Konradt C, Christian DA, Aldridge DL, Bunkofske ME, Jacquet M, Dzierszinski F, Katifori E, Lourido S, Koshy AA, Hunter CA. Nature Microbiology (2025), 10. PMID 40148566.
Intestinal cDC1s provide cues required for CD4+ T cell-mediated resistance to Cryptosporidium. Cohn IS, Wallbank BA , Haskins BE , O’Dea KM, Pardy RD, Shaw S, Merolle MI, Gullicksrud JA, Christian DA, Striepen B, and Hunter CA. Journal of Experimental Medicine (2024), 221(7). PMID 38829369.
Dendritic cell-mediated responses to secreted Cryptosporidium effectors promote parasite-specific CD8(+) T cell responses. Haskins BE, Gullicksrud JA, Wallbank BA, Dumaine JE, Guerin A, Cohn IS, O’Dea KM, Pardy RD, Merolle MI, Shallberg LA, Hunter EN, Byerly JH, Smith EJ, Buenconsejo GY, McLeod BI, Christian DA, Striepen B, Hunter CA. Mucosal Immunology (2024), 17. PMID 38508522.
Immunity to Cryptosporidium: insights into principles of enteric responses to infection. Pardy RD, Wallbank BA, Striepen B, Hunter CA. Nature Reviews Immunology (2024), 24(2). PMID 37697084.
A positive feedback loop controls Toxoplasma chronic differentiation. Licon MH, Giuliano CJ, Chan AW, Chakladar S, Eberhard JN, Shallberg LA, Chandrasekaran S, Waldman BS, Koshy AA, Hunter CA, Lourido S. Nature Microbiology (2023), 8(5). PMID 37081202.

Director, Hunter Laboratory
Christopher A. Hunter, PhD
Mindy Halikman Heyer Distinguished Professor of Pathobiology
Join Us Today
We are always seeking highly motivated students and post-doctoral fellows.
Find Us
University of Pennsylvania
School of Veterinary Medicine
3800 Spruce Street
Philadelphia, PA 19104-4539
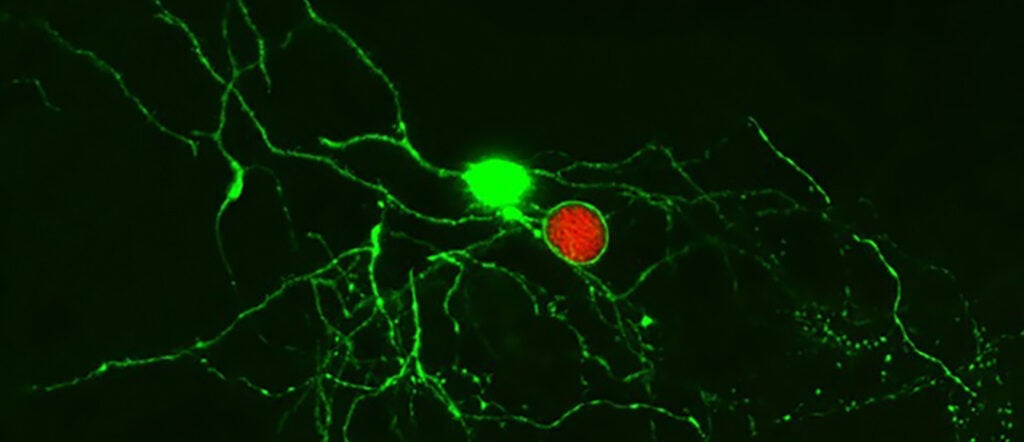
Understanding the immune response to a persistent pathogen (link is external)
Penn Vet researchers show that the immune system can recognize and control the latent stage of the parasite Toxoplasma gondii, a finding that can inform the study of latency in…

A hopeful time for Cryptosporidium research (link is external)
Boris Striepen of Penn Vet organized the First Biennial Cryptosporidium Meeting, bringing together researchers and clinicians from around the world to discuss the problems and progress around the parasite and…
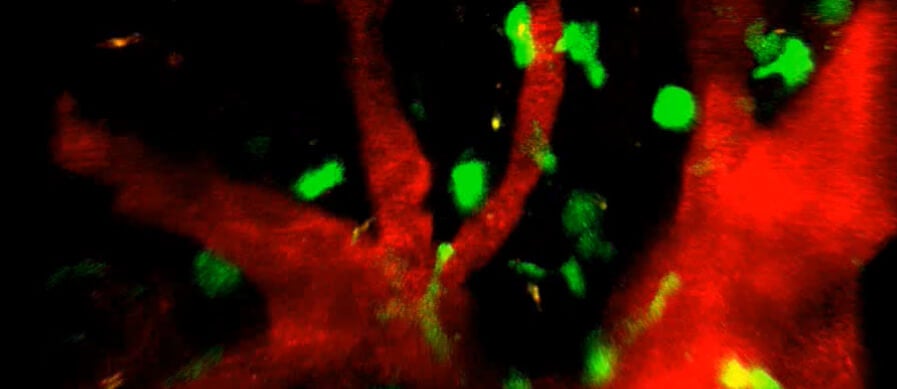
Regulating the regulators of the immune system (link is external)
Research led by Penn Vet scientists reveals a new layer of complexity with which the immune system finds a balance between controlling pathogens and protecting healthy tissue.
