Hunter Laboratory
About Us
For nearly 25 years, the Hunter Laboratory has focused on understanding how the immune response to Toxoplasma gondii is regulated to allow the development of protective immunity as well as to limit T cell mediated pathology in multiple sites including the gut and brain.
Our Research
Christopher Hunter has been working on various aspects of basic parasitology since 1984 and for the last 25 years there has been a focus on understanding how the protective immune response to Toxoplasma gondii develops and how this relates to other parasitic infections.
The Hunter Laboratory team has focused on the innate events that lead to the development of long-term protective immunity mediated by T and B cells. These studies led us to develop expertise in cytokine biology and, while the focus has been in understanding their role in infectious disease, these findings are frequently relevant to cytokine function in autoimmunity and inflammatory processes associated with human disease.
For example, as part of studies to understand how IL-12 family members affect immunity to the T. gondii, we showed that IL-27 was important in limiting the T cell-mediated infection-induced inflammation. We have defined the mechanisms used by IL-27 to influence the immune system and our work has been shown to be relevant to inflammatory processes in multiple experimental systems that includes other infections as well as models of auto-immune inflammation, asthma and cancer.
Since toxoplasma causes a chronic infection in the brain there has been a long-term interest in the neuropathogenesis of infectious diseases and how lymphocytes access and operate in this immune privileged site.
In this laboratory we have developed all of the skills required for the routine analysis of multiple innate immune parameters and to quantify DC, macrophage, NK, T, and B cell responses to infection.
We are also able to utilize different combinations of transgenic parasites (replication deficient, expressing fluorescent reporters, distinct model antigens OVA and E and the Cre recombinase) and TCR transgenic T cells to provide higher resolution analysis of individual parasite specific CD4 and CD8 T cell populations and apply multi-photon microscopy to image the innate and adaptive response to T. gondii.
As part of this research program Dr. Hunter has supervised more than 50 students from multiple programs that include Penn undergraduates, VMD summer students, graduate students from the Microbiology and Immunology graduate groups as well as combined degree (VMD/PhD and MD/PhD), and PhD candidates and undergraduates from the UK. He has been responsible for the training of 15 post-docs and 26 students (20 PhD, three Master’s and three in training).
Almost all of these individuals are still in science with trainees in faculty positions at the University of Washington, UPenn, UVA, Columbia, Yale, UMass, UC Riverside, University of Arkansas and six in faculty positions in Canada, Brazil, Japan and Europe, with several in industry (Pfizer, Medimmune), Science policy at Stanford and a Program Advisor to the Malaria Vaccine Initiative.
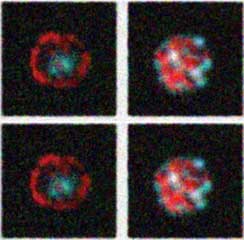
Stylized images of CD8+ T cells looking at differential localization of the transcription factor T-bet in mouse cells after infection with the parasite Toxoplasma gondii, using the Amnis ImageStream. In the cell in the first and third box, T-bet (red) does not co-localize with DAPI (blue), indicating that it is cytoplasmic, whereas in the cell in the second and fourth box, T-bet does co-localize with DAPI, indicating that it is nuclear. Gretchen Harms and David Christian provided these images.

Image stream analysis of STAT6 activation in cells from infected mice. Zsgreen1+ macrophages that contain a single intracellular parasite (top row) or which are uninfected (lower row) are shown. Cells were stained with DAPI to identify the host cell nucleus as well as with a p-STAT6 antibody. The bright field overlay shows that in both cells the pSTAT6 is localized in the host nucleus.
Publications
Immunity to Cryptosporidium: insights into principles of enteric responses to infection. Pardy RD, Wallbank BA, Striepen B, Hunter CA. Nat Rev Immunol. 2024 Feb;24(2):142-155. doi: 10.1038/s41577-023-00932-3. Epub 2023 Sep 11.PMID: 37697084
Development of a nucleoside-modified mRNA vaccine against clade 2.3.4.4b H5 highly pathogenic avian influenza virus. Furey C, Scher G, Ye N, Kercher L, DeBeauchamp J, Crumpton JC, Jeevan T, Patton C, Franks J, Rubrum A, Alameh MG, Fan SHY, Phan AT, Hunter CA, Webby RJ, Weissman D, Hensley SE. Nat Commun. 2024 May 23;15(1):4350. doi: 10.1038/s41467-024-48555-z.PMID: 38782954
A positive feedback loop controls Toxoplasma chronic differentiation. Licon MH, Giuliano CJ, Chan AW, Chakladar S, Eberhard JN, Shallberg LA, Chandrasekaran S, Waldman BS, Koshy AA, Hunter CA, Lourido S. Nat Microbiol. 2023 May;8(5):889-904. doi: 10.1038/s41564-023-01358-2. Epub 2023 Apr 20. PMID: 37081202
The role of macrophages in protective and pathological responses to Toxoplasma gondii.
Park J, Hunter CA.Parasite Immunol. 2020 Jul;42(7):e12712. doi: 10.1111/pim.12712. Epub 2020 Apr 8.PMID: 32187690

Director, Hunter Laboratory
Christopher A. Hunter, PhD
Mindy Halikman Heyer Distinguished Professor of Pathobiology
Join Us Today
We are always seeking highly motivated students and post-doctoral fellows.
Find Us
University of Pennsylvania
School of Veterinary Medicine
3800 Spruce Street
Philadelphia, PA 19104-4539
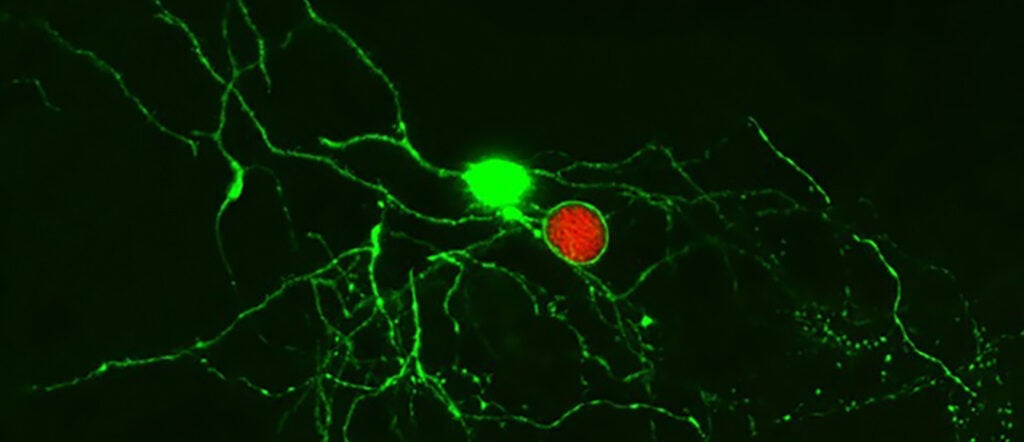
Understanding the immune response to a persistent pathogen (link is external)
Penn Vet researchers show that the immune system can recognize and control the latent stage of the parasite Toxoplasma gondii, a finding that can inform the study of latency in…

A hopeful time for Cryptosporidium research (link is external)
Boris Striepen of Penn Vet organized the First Biennial Cryptosporidium Meeting, bringing together researchers and clinicians from around the world to discuss the problems and progress around the parasite and…
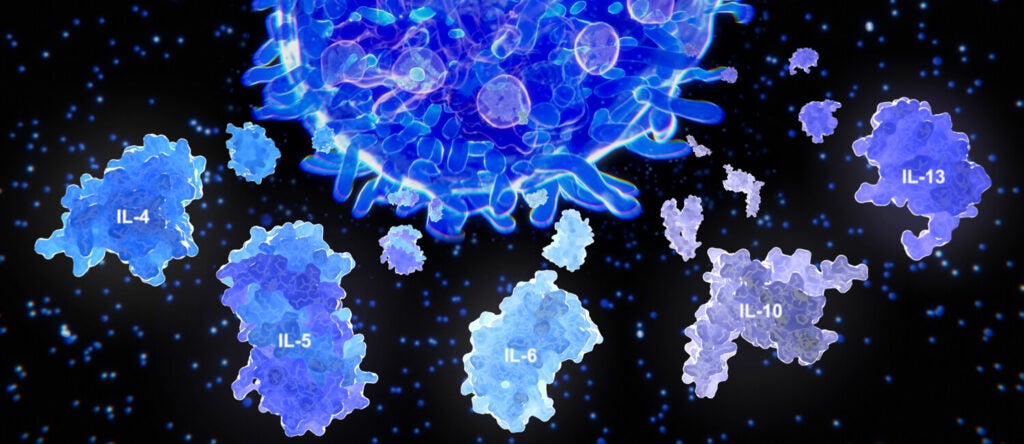
Navigating cytokine storms (link is external)
It’s a trajectory followed by many who experience a severe case of COVID-19: They feel poorly for a few days, improve over a day or two and then, a week…
