Volk Laboratory
The goals of the Volk laboratory are to understand regulatory mechanisms governing dynamic interactions between cells and their surrounding extracellular matrix in the wound healing-fibrosis-cancer progression triad and to apply this knowledge to develop innovative regenerative and oncologic therapies for veterinary and human patients.
Major Projects
Defining a role for type III Collagen (Col3) in regenerative and tumor microenvironments
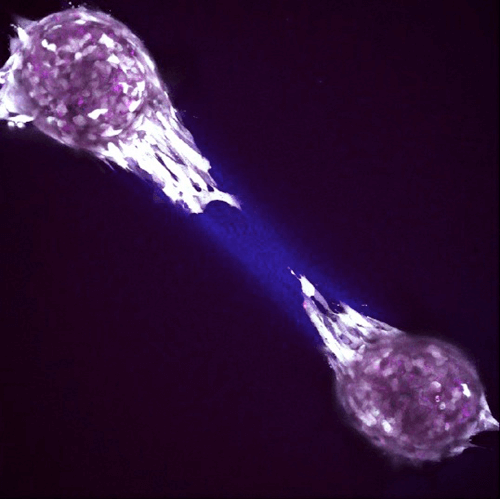
Volk Laboratory publications have characterized a key role for Col3 in directing cellular activities and fate in regenerative and tumor microenvironments.
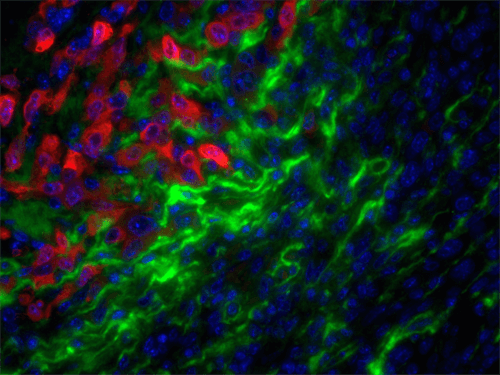
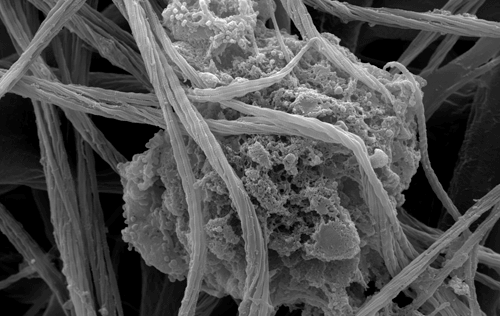
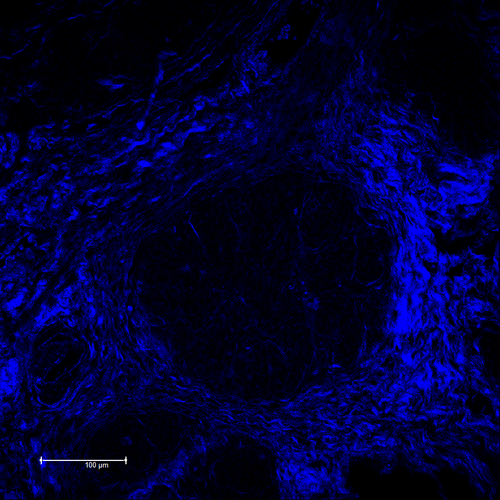
Although Col3 has been widely speculated to play a role in wound healing due to its temporospatial pattern of expression following injury, our studies were the first to reveal a role for Col3 in 1) regulating reepithelialization and scar formation during cutaneous wound healing and 2) modulating bone formation during development and fracture repair. These studies have important implications for the development of novel strategies to target major medical issues such as chronic non-healing wounds, disorders of excessive scar formation, impaired bone healing and osteoporosis. Our study showing that Col3-deficiency promotes scar deposition through the promotion of myofibroblast density and persistence following injury has led to a paradigm shift in the understanding of how individual collagens can suppress scar formation/fibrosis rather than simply contributing to the process. Ongoing studies seek to define mechanisms by which Col3 regulates cell activities and fate and apply this knowledge to the development of novel biomaterials for tissue engineering strategies.
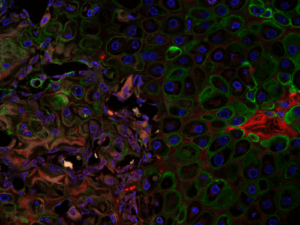
As tumors have been referred to as “wounds that do not heal”, our recent publication identifying a role for Col3 in suppressing breast cancer growth and metastasis by its ability to direct stromal organization complements our wound healing studies. Current studies will determine mechanisms by which Col3 regulates the switch between a tumor-restrictive and tumor-permissive stroma and address the hypothesis that Col3-based regenerative strategies can effectively limit recurrence of certain cancers.
Regenerative therapies for cutaneous wound healing
The extremes in response to cutaneous injury, both non-healing chronic wounds and pathologic scar formation, are a significant cause of morbidity and health care expenditures. Together with collaborators, Volk Laboratory has shown regenerative medical strategies (cell-based therapies, electrical stimulation and biomaterials) improve quality of cutaneous wound healing, and determined mechanisms by which they promote optimal repair, using small and large animal models as well as in human subjects.
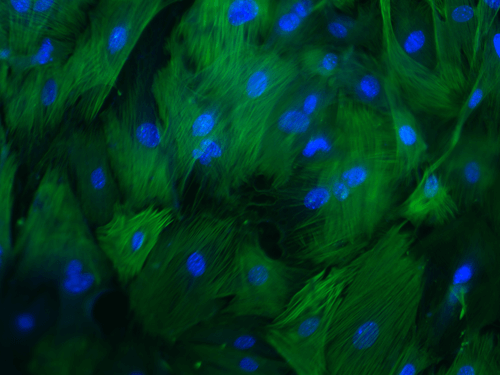
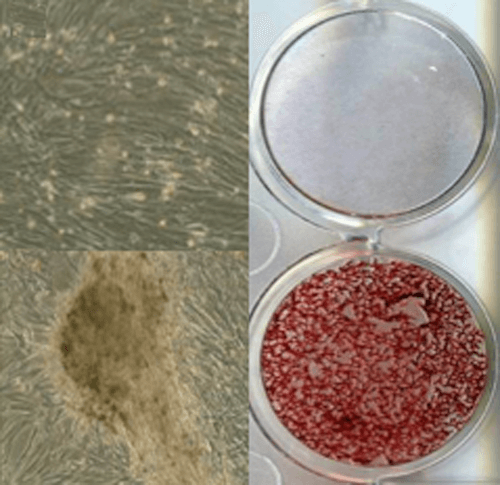
Cellular-based therapies to improve tissue repair in canine models
Our laboratory has a long-standing interest in adult cell-based therapies, with a goal to develop and then optimize novel treatments to improve healing of companion animals. Studies in the Volk Laboratory have focused on advancing a thorough, systematic understanding of the basic biology of canine MSCs to inform the design of future stem cell clinical trials in the dog and their use for translational studies. As spontaneous large animal models play a key role in defining efficacy of regenerative medical therapies, these early studies have provided critical data for model development. Our current clinical trial in client-owned dogs with elbow dysplasia will compare efficacy of post-operative cell-based therapies (bone marrow vs adipose-derived) in improving clinical outcomes.
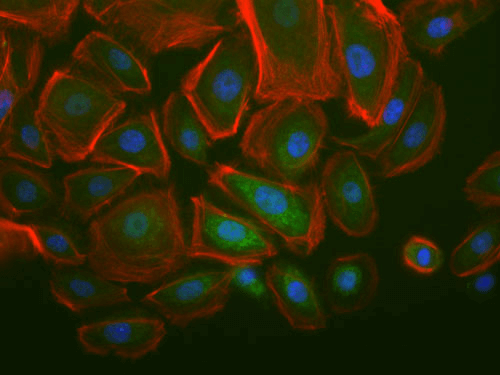
Stromal targets in cancer of companion animals
Studies from a large number of laboratories, including our own, have revealed a critical role for the tumor stroma in controlling cancer behavior. As aggressive stromal signatures predict metastasis and survival in both murine breast cancer models and human patients, we predict these signatures may provide similar prognostic information in dogs and cats with mammary gland tumors. Mammary gland tumors, the most common malignancies in intact female dogs and the third most common in female cats, represent a significant canine health problem. Two major obstacles limit our care of dogs and cats with mammary carcinoma: 1) accurate identification of patients at high-risk of local recurrence and/or metastasis and 2) effective therapies for at risk individuals.
In collaboration with Karin Sorenmo, Director of the Penn Vet Shelter Canine Mammary Tumor Program, and Ellen Pure, Director, Penn Vet Cancer Center, University of Pennsylvania School of Veterinary Medicine and an internationally-recognized expert in the field of the tumor microenvironment, our ongoing studies aim to:
- Identify stromal signatures that predict recurrence/clinical outcomes in dogs and cats with mammary gland tumors to avoid over-treatment of low-risk patients as well as under-treatment of those that require early, aggressive intervention
- Determine mechanisms driving formation of a tumor permissive stroma
- Develop safer, more effective therapies for patients with mammary tumors
Although our initial studies have focused on canine mammary tumors, we have also begun applying our findings to cancers in cats as well. As spontaneously occurring canine and feline mammary carcinomas share many similarities with human breast cancer, our findings may have direct application to human breast cancer research, thus helping both dogs, cats, and women with breast cancer.
Publications
Cysteine-rich domain of type III collagen N-propeptide inhibits fibroblast activation by attenuating TGFb. Brisson BK, Stewart DC, Burgwin C, Chenoweth D, Wells RG, Adams SL, Volk SW*. Matrix Biology. 2022; 109: 19-33. doi: 10.1016/j.matbio.2022.03.004.PMID: 35339637. PMCID: PMC9086147.
Collagen molecular phenotypic switch between non-neoplastic and neoplastic canine mammary tissues. Terajima M, Taga Y, Brisson BK, Durham AC, Sato K, Uzawa K, Saito T, Hattori S, Sørenmo KU, Yamauchi M, Volk SW. Sci Reports. 2021; 11(1): 8659.Doi: 10.1038/s41598-021-87380-y.PMID:33883562.PMCID: PMC8060395.
Cell morphology and mechanosensing can be decoupled in fibrous microenvironments and identified using artificial neural networks. Bonnevie ED, Ashinsky BG, Dekky B, Volk SW, Smith HE, Mauck RL. Sci Reports. 2021 Mar 15;11(1):5950. doi: 10.1038/s41598-021-85276-5. PMID: 33723274.PMCID:PMC7961147.
Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Witherel CE, Sao K, Brisson BK, Han B, Volk SW, Petrie RJ, Han L, Spiller KL. Biomaterials. 269:120667. doi: 10.1016/j.biomaterials.2021.120667. PMCID: PMC7870567.
Intratumoral collagen signatures predict clinical outcomes in feline mammary carcinoma. Rosen SG, Brisson BK, Durham AC, Munroe CM, McNeill CJ, Stefanovski D, Sorenmo KU, Volk SW*. PLoS One,15(8): e0236516 doi: 10.1371/journal.pone.0236516, 2020. PMCID: PMC7416937.

Director, Volk Laboratory
Susan W. Volk, VMD, PhD
Corinne R. and Henry Bower Professor of Surgery
Join Us Today

We are always seeking highly motivated students and post-doctoral fellows.
Find Us
University of Pennsylvania
School of Veterinary Medicine
3800 Spruce Street
Philadelphia, PA 19104-4539

Dr. Susan W. Volk, Named Corinne R. and Henry Bower Professor of Surgery at Penn Vet
Andrew M. Hoffman, DVM, DVSc, DACVIM, Gilbert S. Kahn Dean of the School of Veterinary Medicine at the University of Pennsylvania (Penn Vet) has named Susan W. Volk, VMD, PhD,…