Comparative Immunotherapy Program
Overview
Our Mission
The mission of the Comparative Immunotherapy Program is to improve the lives of both animals and humans by leveraging comparative medicine, to understand immune mechanisms of disease and create innovative immunotherapies in areas of unmet need.
Our Vision
The Comparative Immunotherapy Program’s vision is to foster cross-disciplinary collaborations and facilitate translational research in comparative immunology across departments, schools, and institutions to accelerate the implementation of cutting edge immunotherapies for companion animals and humans.
What is Immunotherapy?
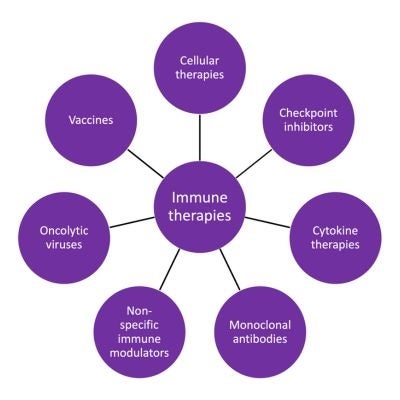
Immunotherapies can be used to treat different diseases either by activating the immune system or suppressing it. Diseases such as cancer and infection can be treated by activating the immune system, enhancing its ability to eliminate cancerous cells or pathogens. Conversely, autoimmunity, inflammation, and organ rejection after transplant can be treated or prevented by suppressing the immune system. There are different types of immunotherapies that affect the immune system in different ways.
These therapies use immune cells, most commonly white blood cells known as T cells, NK cells, or iNKT cells. Other cell types, such as regulatory or suppressor T cells, are being explored for their ability to suppress the immune response in situations of organ transplantation or in cases of an over-exuberant immune response, such as excessive inflammation or auto-immunity. Typically these cells are isolated from the patient, genetically engineered outside the body to augment their specificity and function, and then re-administered to the patient. These cells are most commonly used in the treatment of cancer. Other cell types such as regulatory or suppressor T cells are being explored for their ability to suppress the immune response in situations of organ transplantation or in cases of an over-exuberant immune response such as excessive inflammation or auto-immunity. Cell collection (apheresis) from canine patients for cellular therapies is performed by our extracorporeal therapy team.
Monoclonal antibodies that block inhibitory checkpoint signals in T cells are known as checkpoint inhibitors. These antibodies, through their mechanism of action, augment immune responses and have led to significant, durable clinical responses in human patients with cancer. They are also being explored for their ability to augment immune responses against infectious disease such as malaria and HIV.
Cytokines are proteins used by the immune system to communicate between cells and orchestrate immune responses. Cytokine therapy can be used to augment anti-tumor immune responses. Engineering of cellular components such as T cells to secrete cytokines upon activation is a promising strategy being used to increase anti-tumor responses in patients with solid tumors.
Antibodies are proteins that target specific molecules on the surface of host cells, (including canine, feline, and human cells) as well as bacteria, parasites, and fungi. Monoclonal antibodies are amongst the most successful category of immunotherapies and are being actively developed for veterinary patients with diseases such as cancer, infectious disease, and inflammatory disease. Checkpoint inhibitors are most commonly monoclonal antibodies.
Non-specific immune modulators include BCG, IL-2, and STING agonists are used to enhance a non-specific immune response against cancers and/or infectious disease.
Oncolytic viruses are being explored for the treatment of cancer. These viruses aim to primarily infect tumor cells and lead to tumor destruction. Tumor destruction may then expose the immune system to cancer antigens and boost further immune responses.
Vaccines are used to stimulate the immune system to recognize and attach invading pathogens such as bacteria, viruses, parasites. They are also used to stimulate the immune system to fight against cancer cells. A vaccine exposes the immune system to an antigen, which prompts an immune response. This helps the immune system recognize cancer cells as foreign so it can destroy them. Vaccines can be used to both prevent and treat disease.
Clinical Trials

Through our research into comparative immunology and immunotherapy we aim to offer our canine companions the best possible care, while also contributing to the advancement of veterinary science for the benefit of all dogs and their human counterparts.
Publications
Goodman EE, Berjis A, Sheppard NC, Mason NJ, O’Connor RS, Payne AS. FcεRγI promotes canine CD8 chimeric antigen receptor T cell cytotoxicity through a Syk-NF-κB axis. Mol Ther. 2025 Dec 10:S1525-0016(25)01033-0. doi:10.1016/j.ymthe.2025.12.010. Epub ahead of print. PMID: 41376156.
Ryan Tannir, Letitia Chan, Tomasz M. Grzywa, Orlando Arevalo, Alexandra Neeser, Sara Kahn, Austin Cozzone, Arjun Ramamurthi, Lauren Olenick, Nicola J. Mason, Xueyuan Liu, Leyuan Ma. Chemistry-mediated synthetic virus-cell interaction as a generic approach to enhance gene delivery to mammalian cells. Molecular Therapy Advances. 2026. https://doi.org/10.1016/j.omta.2025.201665.
Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: phase 1 trial interim results. Bagley SJ, Logun M, Fraietta JA, Wang X, Desai AS, Bagley LJ, Nabavizadeh A, Jarocha D, Martins R, Maloney E, Lledo L, Stein C, Marshall A, Leskowitz R, Jadlowsky JK, Christensen S, Oner BS, Plesa G, Brennan A, Gonzalez V, Chen F, Sun Y, Gladney W, Barrett D, Nasrallah MP, Hwang WT, Ming GL, Song H, Siegel DL, June CH, Hexner EO, Binder ZA, O’Rourke DM. Nat Med. 2024 May;30(5):1320-1329. doi: 10.1038/s41591-024-02893-z. Epub 2024 Mar 13. PMID: 38480922.
Cryptic MHC-E epitope from influenza elicits a potent cytolytic T cell response. Hogan MJ, Maheshwari N, Begg BE, Nicastri A, Hedgepeth EJ, Muramatsu H, Pardi N, Miller MA, Reilly SP, Brossay L, Lynch KW, Ternette N, Eisenlohr LC. Nat Immunol. 2023 Nov;24(11):1933-1946. doi: 10.1038/s41590-023-01644-5. Epub 2023 Oct 12. PMID: 37828378.
Pigs as Clinically Relevant Models for Synergizing Interventional Oncology and Immunotherapy. Lee J, Boas FE, Duran-Struuck R, Gaba RC, Schachtschneider KM, Comin-Anduix B, Galic Z, Haile S, Bassir A, Chiang J. J Vasc Interv Radiol. 2024 Jun;35(6):809-817.e1. doi: 10.1016/j.jvir.2024.01.005. Epub 2024 Jan 12. PMID: 38219903.
Diagnostic immunohistochemistry of primary and secondary central nervous system neoplasms of dogs and cats. Rissi DR, Miller AD, Demeter EA, Church ME, Koehler JW. J Vet Diagn Invest. 2024 Mar;36(2):153-168. doi: 10.1177/10406387231221858. Epub 2024 Jan 17. PMID: 38234003; PMCID: PMC10929637.
Development and pharmacokinetic assessment of a fully canine anti-PD-1 monoclonal antibody for comparative translational research in dogs with spontaneous tumors. Sho Yoshimoto, Nicholas Chester, Ailian Xiong, Enrico Radaelli, Hong Wang, Marc Brillantes, Gayathri Gulendran, Patrick Glassman, Don L. Siegel & Nicola J. Mason (2023), mAbs, 15:1, DOI: 10.1080/19420862.2023.2287250
Checkpoint kinase 2 controls insulin secretion and glucose homeostasis. Chong ACN, Vandana JJ, Jeng G, Li G, Meng Z, Duan X, Zhang T, Qiu Y, Duran-Struuck R, Coker K, Wang W, Li Y, Min Z, Zuo X, de Silva N, Chen Z, Naji A, Hao M, Liu C, Chen S. Nat Chem Biol. 2023 Nov 9. doi: 10.1038/s41589-023-01466-4. Epub ahead of print. PMID: 37945898. https://pubmed.ncbi.nlm.nih.gov/37945898/
Unedited allogeneic iNKT cells show extended persistence in MHC-mismatched canine recipients. Rotolo A, Whelan EC, Atherton MJ, Kulikovskaya I, Jarocha D, Fraietta JA, Kim MM, Diffenderfer ES, Cengel KA, Piviani M, Radaelli E, Duran-Struuck R, Mason NJ. Cell Rep Med. 2023 Oct 17;4(10):101241. doi: 10.1016/j.xcrm.2023.101241. PMID: 37852175; PMCID: PMC10591065. https://pubmed.ncbi.nlm.nih.gov/37852175/
PD-L1 positive astrocytes attenuate inflammatory functions of PD-1 positive microglia in models of autoimmune neuroinflammation. Linnerbauer M, Beyer T, Nirschl L, Farrenkopf D, Lößlein L, Vandrey O, Peter A, Tsaktanis T, Kebir H, Laplaud D, Oellinger R, Engleitner T, Alvarez JI, Rad R, Korn T, Hemmer B, Quintana FJ, Rothhammer V. Nat Commun. 2023 Sep 9;14(1):5555. doi: 10.1038/s41467-023-40982-8. PMID: 37689786; PMCID: PMC10492803. https://pubmed.ncbi.nlm.nih.gov/37689786/
Initial investigation of molecular phenotypes of airway mast cells and cytokine profiles in equine asthma. Woodrow JS, Hines M, Sommardahl C, Flatland B, Lo Y, Wang Z, Sheats MK, Lennon EM. Front Vet Sci. 2023 Jan 11;9:997139. doi: 10.3389/fvets.2022.997139. PMID: 36713876; PMCID: PMC9875299. https://pubmed.ncbi.nlm.nih.gov/36713876/
Esomeprazole induces structural changes and apoptosis and alters function of in vitro canine neoplastic mast cells. Gould EN, Szule JA, Wilson-Robles H, Steiner JM, Lennon EM, Tolbert MK. Vet Immunol Immunopathol. 2023 Feb;256:110539. doi: 10.1016/j.vetimm.2022.110539. Epub 2022 Dec 22. PMID: 36592548. https://pubmed.ncbi.nlm.nih.gov/36592548/
Comparative oncology reveals DNMT3B as a therapeutic vulnerability in undifferentiated pleomorphic sarcoma. Fuller AM, DeVine A, Murazzi I, Mason NJ, Weber K, Eisinger KT. Cell Oncol. 2022 Dec; 45(6):1277-1295 doi: 10.1007/s13402-022-00717-1 https://pubmed.ncbi.nlm.nih.gov/36181640/
Generation of non-human primate CAR Tregs using artificial antigen-presenting cells, simian tropic lentiviral vectors, and antigen-specific restimulation. Ellis GI, Deng MZ, Winn DW, Coker KE, Shukla D, Bhoj V, Milone MC, Duran-Struuck R, Riley JL. STAR Protoc. 2022 Nov 5;3(4):101784. doi: 10.1016/j.xpro.2022.101784. PMID: 36386869; PMCID: PMC9641266. https://pubmed.ncbi.nlm.nih.gov/36386869/
cDC1 coordinate innate and adaptive responses in the omentum required for T cell priming and memory. Christian DA, Adams TA 2nd, Shallberg LA, Phan AT, Smith TE, Abraha M, Perry J, Ruthel G, Clark JT, Pritchard GH, Aronson LR, Gossa S, McGavern DB, Kedl RM, Hunter CA. Sci Immunol. 2022 Sep 30;7(75):eabq7432. doi: 10.1126/sciimmunol.abq7432. Epub 2022 Sep 30. PMID: 36179012; PMCID: PMC9835709. https://pubmed.ncbi.nlm.nih.gov/36179012/
Genetic re-direction of canine primary T cells for clinical trial use in pet dogs with spontaneous cancer. Rotolo, A., Atherton, M.J., Kasper, B.T., Haran, K.P., Mason, N.J. STAR Protoc. 2021 Oct 22;2(4):100905. doi: 10.1016/j.xpro.2021.100905. https://pubmed.ncbi.nlm.nih.gov/34746864/
Canine Oncopanel: a capture-based, NGS platform for evaluating the mutational landscape and detecting driver mutations in canine cancers. Wang G., Wu M., Durham AC., Mason NJ., Roth DB. Veterinary and Comparative Oncology. 19 July 2021https://doi.org/10.1111/vco.12746
Cell Clusters Correlate with Submeningeal Pathology in a Natural Model of Multiple Sclerosis. Church ME, Ceja G, McGeehan M, Miller MC, Farias P, Sánchez MD, Swain GP, Assenmacher CA, Stopa EG, Vite CH, Bar-Or A, Alvarez JI. Meningeal B J Immunol. 2021 Jul 1;207(1):44-54. doi: 10.4049/jimmunol.2000514. Epub 2021 Jun 23. PMID: 34162727; PMCID: PMC8695639. https://pubmed.ncbi.nlm.nih.gov/34162727/
Veterinary Cooperative Oncology Group – Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) Following Investigational Therapy in Dogs and Cats. LeBlanc, A., Atherton, M., Bentley, T.R., Boudreau, E., Burton, J., Curran, K., Dow, S., Giuffrida, M., Kellihan, H., Mason, N., Oblak, M., Selmic, L., Selting, K., Singh, A., Tjostheim, S., Vail, D., Weishaar, K., Berger, E., Rossmeisl, J., Mazcko, C. Vet Comp Oncol. 2021 Jun;19(2):311-352. doi: 10.1111/vco.12677. https://pubmed.ncbi.nlm.nih.gov/33427378/
Development of a fully canine anti-canine CTLA4 monoclonal antibody for comparative translational research in dogs with spontaneous tumors. Mason NJ, Chester N., Xiong A., Rotolo A., Wu Y., Glassman P., Gulendran G., Siegel DL. MAbs. Jan-Dec 2021;13(1):2004638.doi: 10.1080/19420862.2021.2004638. https://pubmed.ncbi.nlm.nih.gov/34856888/
Loss of IL-27Rα Results in Enhanced Tubulointerstitial Fibrosis Associated with Elevated Th17 Responses Coppock GM, Aronson LR, Park J, Qiu C, Park J, DeLong JH, Radaelli E, Suszták K, Hunter CA. Loss of IL-27Rα J Immunol. 2020 Jul 15;205(2):377-386. doi: 10.4049/jimmunol.1901463. Epub 2020 Jun 10. PMID: 32522836; PMCID: PMC7368461. https://pubmed.ncbi.nlm.nih.gov/32522836/

Director, Comparative Immunotherapy Program
Nicola J. Mason, BVetMed, PhD, DACVIM, FRCVS
Professor of Medicine and Pathobiology
Support Us
If you would like to support the work we do here please utilize our giving website. We look forward to hearing from you!
Find Us
University of Pennsylvania
School of Veterinary Medicine
3800 Spruce Street
Philadelphia, PA 19104-4539
Allison Ardon
Assistant to the Director

Penn Vet’s Nicola Mason, BVetMed, PhD, DACVIM, FRCVS, Receives Penn Center for Innovation’s Inventor of the Year Award
The University of Pennsylvania (Penn) School of Veterinary Medicine (Penn Vet) congratulates Dr. Nicky Mason, the Paul A. James and Charles A. Gilmore Endowed Chair Professor and Professor of Medicine…

Dogs with cancer are helping save lives—both canine and human (link is external)
The Comparative Immunotherapy Program led by Penn Vet’s Nicola Mason is redefining how therapies are developed and tested—uniting human and veterinary medicine to move promising immunotherapies forward.

Study shows promise for iNKT cell platform to treat cancer (link is external)
Researchers from the School of Veterinary Medicine and Perelman School of Medicine have shown that invariant natural killer T cells from a healthy donor can persist in MHC-mismatched canines, demonstrating…
