Our Research
Latest Research
In the mammalian soma, tissue-specific stem cells capable of maintaining the proliferative output necessary for tissue organization and function exist in a state of multipotency (the ability to generate any cell type of that particular tissue, in contrast to the pluripotent state embodied by embryonic stem cells capable of generating all cell types of the mammalian organism). In highly proliferative tissues such as the epithelial lining of the intestine, data from our lab and others has begun to establish a model in the stem cell compartment is organized into a hierarchy, with a mostly dormant population of long-live, radio-resistant reserve stem cells at the top of this hierarchy. When activated, these reserve stem cells give rise to a second, highly proliferative, radiosensitive short-term stem cell that bears the daily proliferative burden required to maintain tissue homeostasis.
Our lab is focused on understanding the relationship between these two stem cell populations, the molecular determinants of reserve intestinal stem cell activation, and how deregulation of the reserve intestinal stem cell compartment contributes to disease states such as colorectal cancer or acute gastrointestinal radiation injury.
We have recently identified the Msi family of RNA binding proteins as potent oncoproteins in both hematopoietic and intestinal malignancies. Msi proteins are expressed in putative somatic stem cell compartments, are frequently found to be overexpressed in advanced cancers, and are known to govern asymmetric cell division in Drosophila melanogaster (a process thought to maintain the somatic stem cell niche in mammals). Using mouse genetic approaches integrated with human patient data, we have recently demonstrated that MSI2 acts as an intestinal oncogene, driving activation of the mTORC1 complex and uncontrolled stem cell expansion. We are currently pursuing the role of Msi proteins in epithelial stem cell compartments using tissue-specific gene ablation and drug-inducible gene activation. The effects of Msi proteins on stem cell maintenance and oncogenic transformation are being tied to their RNA binding capacity using CLIP-Seq analysis (immunoprecipitation of Msi-interacting RNAs followed by massively parallel sequencing) in order to determine how specific Msi-RNA interactions affect stem cell self-renewal and oncogenic transformation.
While murine genetic systems are the primary tool of the laboratory, we also work to model human genetic gastrointestinal disorders using induced pluripotent stem (iPS) cells generated from patients. Generation of isogenic diseased and disease-allele corrected iPS cell lines using nuclease-mediated homologous recombination followed by directed differentiation into intestinal tissue provides a controlled platform not only for studying the molecular mechanisms underlying
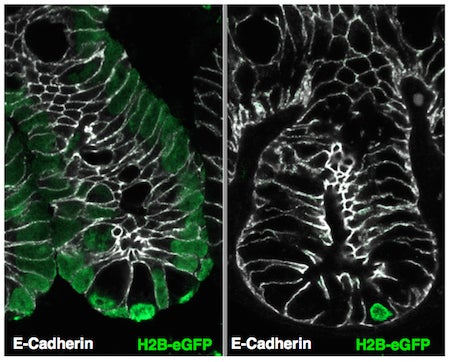
Label retaining cells of the intestinal crypts are identified by loading all cells with a Histone H2B protein fused to a green fluorescent protein (left). Several weeks later, only cells that do not divide retain the fluorescent label in their chromatin.
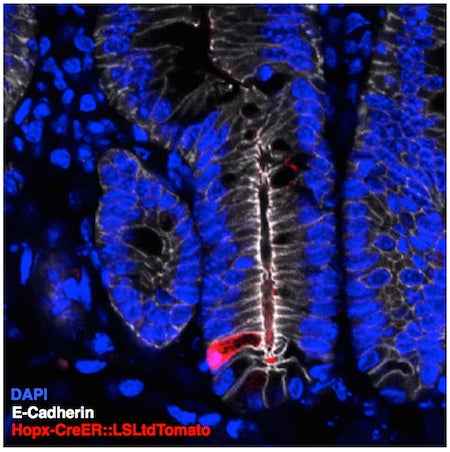
A glimpse of the rare reserve intestinal stem cell (red). This cell is capable of regenerating the entire intestinal lining after injury such as exposure to high doses of radiation.
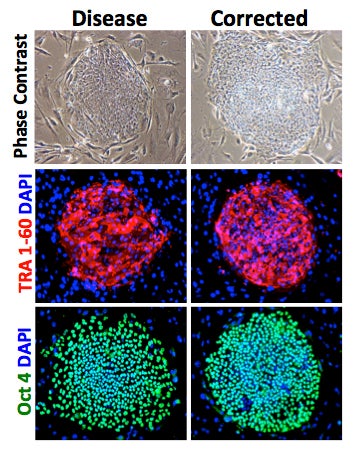
Human induced pluripotent stem cells (iPS) derived from skin cells of a patient with a genetic gastrointestinal disorder followed by genome editing to correct the disease-causing allele. The disease and corrected iPS cultures retain pluripotency assessed by expression of OCT4 and TRA1-6.
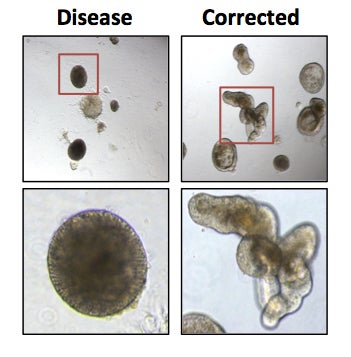
Human intestinal organoids were generated from disease and disease-corrected iPS cell lines. Correction of the disease allele restores the ability of these cells to form budding, branched organoids that recapitulate the crypt structure of the small intestinal epithelium.
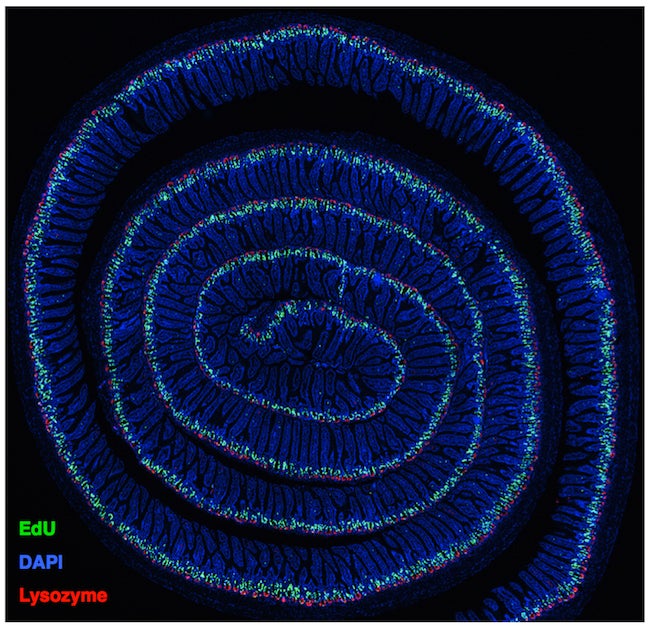
Histological section of the entirety of the murine small intestine. Immunofluorescence staining reveals proliferative cells within crypts (green), Paneth cells at crypt bases (red), and total cell nuclei (blue).
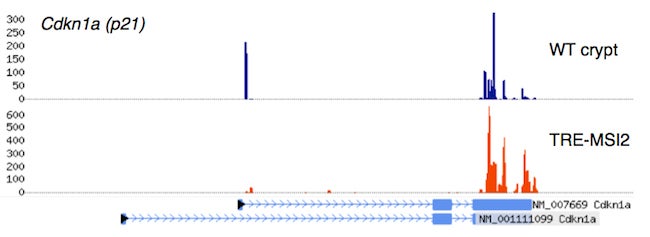
Crosslinking, Immunoprecipitation, and massively parallel sequencing of RNA bound to the Msi2 oncoprotein in wildtype small intestinal crypts, in intestinal epithelium ectopically expressing MSI2 (TRE-MSI2), revealing strong interactions between Msi2/MSI2 and the mRNA encoding the tumor suppressor p21.

Human induced pluripotent stem cells (iPS) derived from skin cells of a patient with a genetic gastrointestinal disorder followed by genome editing to correct the disease-causing allele. The disease and corrected iPS cultures retain pluripotency assessed by expression of OCT4 and TRA1-6.
The Lengner lab frequently employs Southern Blotting. It enables the unequivocal assessment of specific genetic modifications in stem cells and mice.
