Research Newsletter | Spring 2022
FEATURED ARTICLE
Understanding and Enhancing Swine Welfare
Thomas D. Parsons, VMD, PhD, DACAW, was recently named the Marie A. Moore Professor of Animal Welfare and Ethics in the Department of Clinical Studies–New Bolton Center. Dr. Parsons has expertise in swine medicine and behavior and is also the Director of Penn Vet’s Swine Teaching and Research Center.
Dr. Parsons’ experience with pigs started at a young age, as he was raised on a diversified family farm in western Massachusetts that grew both crops and livestock, including pigs. The family farm continues today, and his nephew is the 10th generation of Parsons to work land deeded to their family by King George. It has been said that the second-best thing that a farmer’s son can do is to become a veterinarian—with staying home to take over the farm being the preferred option. Once his brother decided to forgo an academic career as a swine nutritionist and return home to take over the farm, Dr. Parsons was freed to pursue a journey that has taken him around the world, to human medicine and back, and perhaps ironically left him more or less where he started as a child—at home with the pigs.
Dr. Parsons’ experience with pigs started at a young age, as he was raised on a diversified family farm in western Massachusetts that grew both crops and livestock, including pigs. The family farm continues today, and his nephew is the 10th generation of Parsons to work land deeded to their family by King George. It has been said that the second-best thing that a farmer’s son can do is to become a veterinarian—with staying home to take over the farm being the preferred option. Once his brother decided to forgo an academic career as a swine nutritionist and return home to take over the farm, Dr. Parsons was freed to pursue a journey that has taken him around the world, to human medicine and back, and perhaps ironically left him more or less where he started as a child—at home with the pigs.
Dr. Parsons developed an interest in the nervous system as an undergraduate at Amherst College. He was considering a career in medical research when as a junior he discovered Penn’s Veterinary Medical Scientist Training Program and realized he might be able to keep his ties to agriculture while diving into fundamental research. Dr. Parsons went on to receive both his VMD and PhD from Penn. As a veterinary student, Dr. Parsons was required to pay special attention to lectures on pigs so that he would be able to answer his father’s questions. While this laid the foundation for his interest in swine medicine, Dr. Parsons left Penn to pursue post-doctoral training in basic neuroscience. He ended up at the Max Planck Institute for Biomedical Research in Heidelberg, Germany to work with Drs. Wolfhard Almers and Bert Sakmann (the latter of whom won The Nobel Prize in Physiology or Medicine in 1991). It was here that he became fascinated with the auditory system and performed seminal experiments on the unusual synaptic properties of cochlear hair cells.
Dr. Parsons was recruited back to Penn Vet’s Department of Clinical Studies–New Bolton Center to help develop a clinical program in swine medicine and was encouraged to continue his work in the basic sciences. He established a collaboration and later gained laboratory space in the Department of Otorhinolaryngology in the Perelman School of Medicine. Dr. Parsons spent his first ten years as a faculty member pursuing fundamental research on human hearing and deafness. He also worked to establish swine medicine at Penn Vet by dividing his time between the Philadelphia and New Bolton Center campuses. During this time, Dr. Parsons had the opportunity to design and build a new swine facility at Penn Vet. His time spent living in Germany had provided an opportunity to experience a different relationship with food, as European expectations were higher for both the quality of the product as well as how it was raised. Anticipating that these changing views about food would eventually travel across the Atlantic Ocean, Dr. Parsons used the new swine facility for the first study of alternatives to gestation stalls and farrowing crates carried out at an academic institution in the United States (US), thus heralding a new focus on food animal welfare.
Over the next five years, public interest in animal welfare grew in the US and Dr. Parsons began shifting his efforts away from the auditory system to concentrate on swine behavior and welfare; reinventing himself as an applied ethologist. He was instrumental in helping nearly 100 farms across the US transition from stalls to pens with over 200,000 sows being fed with the welfare-friendly management systems originally prototyped at the swine center. He also built one of the largest research groups in the US working on sow housing and welfare.
Dr. Parsons and his collaborators helped establish the concept of personality types in pigs, revealing that these behavioral differences are established early in the pig’s life and robust over time and context. Individual behavioral differences in animals speak to the complexity of animal welfare and has important implications when considering facility design as well as establishing welfare standards. Dr. Parsons’ group was also the first to apply judgement bias tasks to ask questions about gestating sows’ welfare. This experimental paradigm, adapted from human psychology, exploits the tendency of our mood or emotional state to impact our judgements. Judgement bias aims to reveal an animal’s affect state (mood) by determining how sows interpret or judge ambiguous cues. Animals with a positive bias (read optimistic) will treat an ambiguous cue in the same way as known positive cues, whereas pessimistic animals will respond as though the cue was negative. Taken together, this work helps us to better understand the emotional experience of farm animals.
Many unanswered questions remain about pig behavior and welfare. Application of wearable devices (piggy Fitbits) and computerized vision systems supported by artificial intelligence are the current foci of Dr. Parsons’ research team and promise to give us new insights into animal production and welfare. His work fits into a larger rubric of societal concerns about animal agriculture now at the forefront of public discourse. Emerging questions about social justice, diversity, equity, and climate management highlight the complexity of today’s food systems and delineate additional opportunities for improvement in animal agriculture. Penn Vet looks forward to launching a new center in the fall that addresses these challenges. With Dr. Parsons as the head of the new center based at the New Bolton Center campus, its mission aims to make animal agriculture part of the solution to a more livable future.
Latest Research
Highlighted News

Revising the lifecycle of an important human parasite (link is external)
Researchers from Dr. Boris Striepen’s lab tracked Cryptosporidium in real time, creating a new paradigm for how the widespread parasite reproduces in a host.
Correcting night blindness in dogs (link is external)
Researchers in the School of Veterinary Medicine and colleagues have developed a gene therapy that restores dim-light vision in dogs with a congenital form of night blindness, offering hope for…

COVID in a cat (link is external)
A report led by Penn Vet’s Dr. Elizabeth Lennon and colleagues has confirmed what is believed to be the first published account of the delta variant of SARS-CoV-2 in a house…

SARS-CoV-2 is moving between humans and wildlife (link is external)
In humans the pandemic is showing signs of ebbing. In white-tailed deer and other wildlife, however, infections appear widespread.
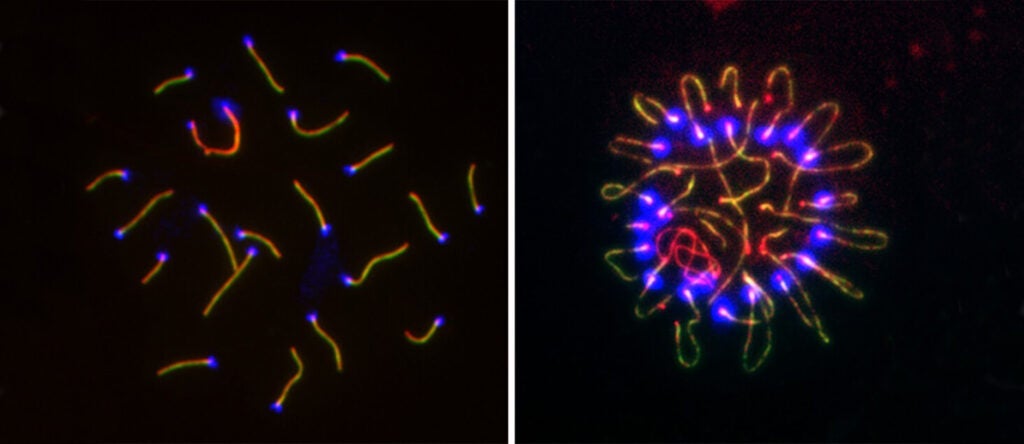
Revealing the mysterious biology of a fundamental process: reproduction (link is external)
New findings from the lab of Dr. P. Jeremy Wang shed light on the cell division process that creates eggs and sperm, recurrent pregnancy loss, and the mechanism by which…
Student Research Day
Penn Vet’s Student Research Day was held on Friday, March 18, 2022, in the Vernon and Shirley Hill Pavilion. Due to the COVID-19 pandemic, this year marked the first time the event had been held in person since 2019. The organizing committee was led by Professor Jennifer Punt and included faculty members Elizabeth Lennon, Michael May, and Phillip Scott, and members of the Student Research Club, Daria Mrugala, Nimisha Pattada, and Stephanie Sila.
Dean Andrew Hoffman kicked off the day’s events with an inspiring introduction focusing on the importance of science and scientists in today’s world. Three VMD students (Jo Kawabata, Amelie Ya Deau, and Hanna Zayas) and three VMD/PhD students (Suna Cranfill, Sabina Hlavaty, and Martha Stone) delivered oral presentations, and 29 posters were presented. Professor Susan VandeWoude, director of the One Health Institute at Colorado State University, delivered a fascinating keynote presentation entitled SARS-CoV-2 infections in felids of all sizes: Implications for human and animal health.
Academic prizes were awarded for three poster presentations (Isabella Healy, Ariel Shepley-McTaggart, and Anna Smith), and all six student oral presentations. This year’s academic prizes for oral and poster presentations were named for Professor Emeritus Richard O. Davies. Faculty judges for the abstracts and posters included Eman Anis, Andres Blanco, Brian Flesner, Jennifer Lenz, Andrew Modzelewski, Lisa Murphy, and Kotaro Sasaki.
Student Research Day also included a ‘poster slam’ competition. Each poster presenter provided a one-minute video and one slide to introduce their poster. These introductions were compiled into a single video that played on a loop in Marookian Auditorium during the poster session. Prizes were awarded in three categories: Clear, Creative, and Crazy. Winners were chosen by popular vote and included Brianna Blunck and Carlin Hagmaier (Clear category); Maria Pansulla and Ariel Shepley-McTaggart (Creative category); and Justin Hoffman (Crazy category).
During the reception, Gayle Joseph, Senior Research Resources Manager, who retired in January 2022, was honored for her tireless advocacy for research at Penn Vet and was recognized for transforming Student Research Day into a vibrant, professional event.
Student Research Day presentations are available for online viewing.
Andrew J. Modzelewski recently joined the department of Biomedical Sciences as an Assistant Professor of Molecular Biology. He received a BSc in Biochemistry and Molecular Biology from Penn State University and earned his PhD in Genetics, Genomics and Development from Cornell University, and just completed a post-doctoral fellowship at University of California–Berkeley. He is interested in understanding the phenomenon of retrotransposon reactivation that is essential in preimplantation embryos and for germline development. He plans to extend this research to understand how defects in these normal biological processes contribute to diseases, cancer and fertility in both humans and animals. To study these, the “ModzLab” develops tools for embryo manipulation (CRISPR-EZ) and single cell measurement (TRI-Blot for combined Western/RT-PCR/DNA) that are already widely adapted for direct and immediate practical application in research and animal production. Outside of the lab, Andrew enjoys hiking, kayaking, snowboarding and restoring antiques.
Honors & Achievements
Three Faculty from the University of Pennsylvania’s School of Veterinary Medicine Appointed to Endowed Professorships
Thomas D. Parsons, Christopher J. Lengner and Amy L. Johnson have been recognized for scholarly achievements.
Dr. Andrew Hoffman Inducted as Fellow of the College of Physicians of Philadelphia
Andrew Hoffman, DVM, DSc, DACVIM, the Gilbert S. Kahn Dean of Veterinary Medicine at the University of Pennsylvania’s School of Veterinary Medicine (Penn Vet) was recognized as a newly elected…
Hunter and Beiting Receive Teaching and Mentoring Awards
Christopher A. Hunter, Mindy Halikman Heyer Distinguished Professor of Pathobiology, has been selected as this year’s recipient of the Michael P. Nusbaum Graduate Student Mentoring Award. This award recognizes Professor Hunter’s caring and dedication as a mentor and his longstanding contributions to the Biomedical Graduate Studies (BGS) community, mentoring students in his own lab, and within the broader BGS parasitology, microbiology, and immunology communities.
Daniel P. Beiting, Assistant Professor, has been selected as this year’s recipient of the Jane M. Glick Graduate Student Teaching Award. This award recognizes excellence in graduate teaching and/or innovation in teaching methods or approaches. Dr. Beiting is well-known throughout Biomedical Graduate Studies (BGS) for his exceptional dedication to teaching of analytical methods to analyze large datasets, and his classes are widely recognized throughout the BGS community as models of excellence in teaching students complex methodologies that they can apply to their own research.
Harty and Freedman Publish “Spotlight Article” in Journal of Virology
Congratulations to Drs. Ronald N. Harty and Bruce D. Freedman whose article, “WWOX-Mediated Degradation of AMOTp130 Negatively Affects Egress of Filovirus VP40 Virus-Like Particles,” was selected as a “Spotlight Article” in Journal of Virology. One of their images (at right) was chosen for the cover of the March 2022 issue of the journal.
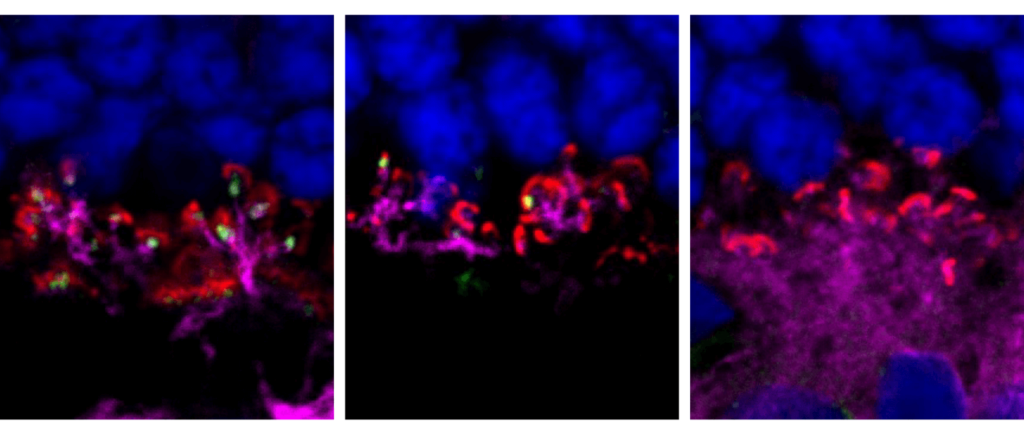
Dr. Bethan Wallbank—2022 Art in Science Competition Winner
Dr. Bethan Wallbank, a postdoctoral researcher in the laboratory of Professor Boris Striepen, won this year’s “Art in Science” competition hosted by the Perelman School of Medicine’s Biomedical Postdoctoral Programs.
Dr. Wallbank provided the following description of the photograph:
This is a fluorescent image of a slice of mouse small intestine infected with the enteric parasite Cryptosporidium parvum. This parasite has adapted to survive in this rapidly turning organ in which it carries out its entire lifecycle. Parasite infection leads to increased turnover of the small intestine. I have demonstrated this by administering EdU, a thymidine analog that incorporates into newly synthesized DNA, to infected mice. The use of a fluorescent label that binds to EdU (shown here in red) identifies newly proliferated cells and their migration out of the crypts. By imaging over the course of infection we can track this migration over time. Chemically and genetically altering epithelial migration allows us to understand the role of intestinal turnover in parasite survival and host defense. Red = EdU (fluorescent azide), green = actin (phalloidin), blue = nuclei (Hoechst).
Select Publications and Grants
Read the latest scholarly publication and grant information from Penn Vet researchers.
A selection of recent publications from Penn Vet faculty:
Arzi B, Webb TL, Koch TG, Volk SW, Betts DH, Watts A, Goodrich L, Kallos MS, and Kol A. Cell Therapy in Veterinary Medicine as a Proof-of-Concept for Human Therapies: Perspectives From the North American Veterinary Regenerative Medicine Association. Front Vet Sci 2021;8:779109.
Asatryan B, Asimaki A, Landstrom AP, Khanji MY, Odening KE, Cooper LT, Marchlinski FE, Gelzer AR, Semsarian C, Reichlin T, Owens AT, and Chahal CAA. Inflammation and Immune Response in Arrhythmogenic Cardiomyopathy: State-of-the-Art Review. Circulation 2021;144:1646-1655.
Callan MB, Thawley VJ, Marryott KA, Shabro A, Fernando S, Kahn S, Hudson KE, and Hod EA. Hemolytic anemia blunts the cytokine response to transfusion of older red blood cells in mice and dogs. Transfusion 2021;61:3309-3319.
Chotiner JY, Leu NA, Xu Y, and Wang PJ. Recurrent pregnancy loss in mice lacking the X-linked Ccnb3 genedagger. Biol Reprod2022;106:382-384.
Cole SD and Rankin SC. Characterization of 2 Klebsiella pneumoniae carbapenemase-producing Enterobacterales isolated from canine rectal swabs. J Vet Diagn Invest 2022;34:306-30.
Dumaine JE, Sateriale A, Gibson AR, Reddy AG, Gullicksrud JA, Hunter EN, Clark JT, and Striepen B. The enteric pathogen Cryptosporidium parvum exports proteins into the cytosol of the infected host cell. Elife 2021;10.
Engiles JB, Uzal FA, Navarro MA, Reef VB, and Bender SJ. Phlegmonous gastritis in 2 yearling horses. J Vet Diagn Invest2022:10406387211065044.
Guan F, You Y, Fay S, Li X, and Robinson MA. Novel Algorithms for Comprehensive Untargeted Detection of Doping Agents in Biological Samples. Anal Chem 2021;93:7746-7753.
Guérin A, Roy NH, Kugler EM, Berry-Sterkers L, Burkhardt JK, Shin J-B, and Striepen B. A Screen for Cryptosporidium Rhoptry Proteins Identifies ROP1 as an Effector Targeting the Host Cytoskeletal Modulator LMO7. Cell Host & Microbe;29:1-14.
Holl HM, Armstrong C, Galantino-Homer H, and Brooks SA. Transcriptome diversity and differential expression in supporting limb laminitis. Vet Immunol Immunopathol 2022;243:110353.
Horback KM and Parsons TD. Judgement bias of group housed gestating sows predicted by behavioral traits, but not physical measures of welfare. PLoS One 2022;17:e0264258.
Jasinski SE, Heckert AB, Sailar C, Lichtig AJ, Lucas SG, and Dodson P. A softshell turtle (Testudines: Trionychidae: Plastomeninae) from the uppermost Cretaceous (Maastrichtian) Hell Creek Formation, North Dakota, USA, with implications for the evolutionary relationships of plastomenines and other trionychids. Cretaceous Research 2022;135.Lau YK, Peck SH, Arginteanu T, Wu M, Lin M, Shore EM, Klein PS, Casal ML, and Smith LJ. Effects of lithium administration on vertebral bone disease in mucopolysaccharidosis I dogs. Bone 2022;154:116237.
Lenz OC, Marques AD, Kelly BJ, Rodino KG, Cole SD, Perera R, Weiss SR, Bushman FD, and Lennon EM. SARS-CoV-2 Delta Variant (AY.3) in the Feces of a Domestic Cat. Viruses 2022;14.
Liang J, Ruthel G, Freedman BD, and Harty RN. WWOX-Mediated Degradation of AMOTp130 Negatively Affects Egress of Filovirus VP40 Virus-Like Particles. J Virol 2022;96:e0202621.
Lilly ML, Watson B, and Siracusa C. Behavior Education and Intervention Program at a Small Shelter I. Effect on Behavior Knowledge and Safety. J Appl Anim Welf Sci 2021:1-13.
Liu R, Kasowitz SD, Homolka D, Leu NA, Shaked JT, Ruthel G, Jain D, Lin H, Keeney S, Luo M, Pillai RS, and Wang PJ. YTHDC2 is essential for pachytene progression and prevents aberrant microtubule-driven telomere clustering in male meiosis. Cell Reports 2021;37:110110.
Lund M, Mauldin EA, Radaelli E, and Bradley CW. Palisading granulomatous dermatitis and panniculitis (palisading granuloma) of dogs. Vet Pathol 2021;58:1091-1099.
Milne EM, Piviani M, Hodgkiss-Geere HM, Piccinelli C, Cheeseman M, Cazzini P, Ressel L, Marcos RJ, Marrinhas CS, Santos MS, Thomas EK, Drummond D, and Martinez Pereira Y. Comparison of effusion cell block and biopsy immunohistochemistry in mesothelial hyperplasia, mesothelioma, and carcinoma in dogs. Vet Clin Pathol 2021;50:555-567.
Miyadera K, Santana E, Roszak K, Iffrig S, Visel M, Iwabe S, Boyd RF, Bartoe JT, Sato Y, Gray A, Ripolles-Garcia A, Dufour VL, Byrne LC, Flannery JG, Beltran WA, and Aguirre GD. Targeting ON-bipolar cells by AAV gene therapy stably reverses LRIT3-congenital stationary night blindness. Proc Natl Acad Sci U S A 2022;119:e2117038119.
Ozturk BE, Johnson ME, Kleyman M, Turunc S, He J, Jabalameli S, Xi Z, Visel M, Dufour VL, Iwabe S, Pompeo Marinho LFL, Aguirre GD, Sahel JA, Schaffer DV, Pfenning AR, Flannery JG, Beltran WA, Stauffer WR, and Byrne LC. scAAVengr, a transcriptome-based pipeline for quantitative ranking of engineered AAVs with single-cell resolution. Elife 2021;10.
Powell L, Houlihan C, Stone M, Gitlin I, Ji X, Reinhard CL, and Watson B. Animal Shelters’ Response to the COVID-19 Pandemic: A Pilot Survey of 14 Shelters in the Northeastern United States. Animals (Basel) 2021;11.
Powell L, Stefanovski D, Siracusa C, and Serpell J. Owner Personality, Owner-Dog Attachment, and Canine Demographics Influence Treatment Outcomes in Canine Behavioral Medicine Cases. Front Vet Sci 2020;7:630931.
Rurik JG, Tombacz I, Yadegari A, Mendez Fernandez PO, Shewale SV, Li L, Kimura T, Soliman OY, Papp TE, Tam YK, Mui BL, Albelda SM, Puré E, June CH, Aghajanian H, Weissman D, Parhiz H, and Epstein JA. CAR T cells produced in vivo to treat cardiac injury. Science 2022;375:91-96.
Singh TP, Carvalho AM, Sacramento LA, Grice EA, and Scott P. Microbiota instruct IL-17A-producing innate lymphoid cells to promote skin inflammation in cutaneous leishmaniasis. PLoS Pathog 2021;17:e1009693.
Stavisky J, Watson B, Dean R, Merritt BL, van der Leij R, and Serlin R. Educational Research Report Development of International Learning Outcomes for Shelter Medicine in Veterinary Education: A Delphi Approach. J Vet Med Educ2021;48:610-619.
Tadlock S, Phillips CA, Casal ML, Kraus MS, Gelzer AR, and Boland MR. Development of an Informatics Algorithm to Link Seasonal Infectious Diseases to Birth-Dependent Diseases Across Species: A Case Study with Osteosarcoma. AMIA Jt Summits Transl Sci Proc 2021;2021:585-594.
Wang Z, Cheong MC, Tsien J, Deng H, Qin T, Stoltzfus JD, Jaleta TG, Li X, Lok JB, Kliewer SA, and Mangelsdorf DJ. Characterization of the endogenous DAF-12 ligand and its use as an anthelmintic agent in Strongyloides stercoralis. Elife2021;10.
Yan F, Li J, Milosevic J, Petroni R, Liu S, Shi Z, Yuan S, Reynaga JM, Qi Y, Rico J, Yu S, Liu Y, Rokudai S, Palmisiano N, Meyer SE, Sung PJ, Wan L, Lan F, Garcia BA, Stanger BZ, Sykes DB, and Blanco MA. KAT6A and ENL Form an Epigenetic Transcriptional Control Module to Drive Critical Leukemogenic Gene-Expression Programs. Cancer Discov 2022;12:792-811.
Yang F, Gracia Gervasi M, Orta G, Tourzani DA, De la Vega-Beltran JL, Ruthel G, Darszon A, Visconti PE, and Wang PJ. C2CD6 regulates targeting and organization of the CatSper calcium channel complex in sperm flagella. Development 2022;149.
Lillian Aronson
Nestlé Purina
Untargeted plasma metabolomics and biomarker, DNA, and microbiome assessments in cats with and without chronic kidney disease
1/1/22—12/31/22
$119,373
Michael Atchison
NIH (competitive renewal)
VMD-PhD training in infectious disease-related research
7/1/21—6/30/22
$202,096
Matthew Atherton
NIH
Prognostic and therapeutic implications of IFNAR1 signaling on CAR T cell therapy for cancer
7/1/21—6/30/26
$711,345
William Beltran
Foundation for Fighting Blindess
Preclinical evaluation of an iPSC-derived photoreceptor therapeutic in canine model of retinitis pigmentosa
1/1/22—12/31/22
$250,000
Andres Blanco
URF
Investigating the role of KAT6A as a potential therapeutic target in acute myeloid leukemia
3/1/22—2/28/23
$50,000
Andres Blanco
Internal
Dual targeting of LSD1 and KAT6A to induce therapeutic differentiation in AML
7/1/21—6/30/22
$50,000
Andres Blanco
Margaret Q. Landenberger Research Foundation
Investigating the role of KAT6A as a differentiation therapy target MLL-rearranged acute myeloid leukemia
11/1/21—10/31/23
$100,000
Andres Blanco
American Association for Cancer Research
Evaluating KAT6A as a novel target for non-APL AML differentiation therapy
12/1/21—11/30/22
$25,000
Zhengxia Dou
National Institute of Food and Agriculture/Department of Agriculture
Developing novel feeds via bioprocessing of food waste and crop residue biomass to support sustainable dairy production
2/1/22—1/31/25
$769,231
Brian Flesner
Companion Animal Research Fund (internal)
Use of the PI3K inhibitor copanlisib to treat splenic hemangiosarcoma harboring PI3K pathway mutations
4/15/22—4/15/24
$30,000
Brian Flesner
Curebiotech
Evaluation of Resiquimod (CB101) in combination with radiation therapy in dogs with head & neck cancer
2/9/22—2/9/23
$31,147
Maureen Griffin
Companion Animal Research Fund (internal)
Cathepsin-activated near-infrared (NIR) imaging for intraoperative detection of insulinomas
4/1/22—4/1/24
$29,093
Maureen Griffin
Companion Animal Research Fund (internal)
Evaluation of pre- and intra-operative sentinel lymph node mapping techniques for canine thyroid carcinoma
4/1/22—4/1/24
$29,907
Jennifer Lenz
Companion Animal Research Fund (internal)
Exploring the osteopontin-CD44 axis to uncover therapeutic targets for histiocytic sarcoma
7/1/22—7/1/24
$29,945
Claudia Lovell (Anguera Lab)
Lupus Foundation of America, Philadelphia Tri-State Chapter
Defining X chromosome inactivation and escape in age-associated B cells
7/1/21—6/30/22
$3,000
Claudia Lovell (Anguera Lab)
Lupus Foundation of America, Inc.
Defining X chromosome inactivation maintenance and escape in age-associated B cells
6/30/21—12/31/21
$4,000
Claudia Lovell (Anguera Lab)
Rheumatology Research Foundation
Future Physician Scientist Award
7/1/21—6/30/23
$30,000
Nicola Mason
NIH
Coordinating Center for Canine Immunotherapy Trials and Correlative Studies
9/1/21—8/31/22
$349,529
Andrew Modzelewski
Eunice Kennedy Shriver National Institute of Child Health & Human Development
The role of retrotransposon activity in mammalian pre-implantation development
3/18/22—1/31/23
$153,230
Kyla Ortved
University of Wisconsin-Madison
Translational research workforce training: Leveraging the veterinary specialist
8/1/21—7/31/22
$76,893
Cynthia Otto
NIH
Effective, reagent-free detection of the odor signature of COVID-19 infection using a nano-enabled sensor array
1/10/2022—12/31/2022
$52,810
Mark Oyama
CHOP/R01
Serotonin signaling in mitral valve homeostasis, maintenance and restoration
3/1/22—2/28/23
$2,114
Mark Oyama
Morris Animal Foundation
Comparative Medicine and Translational Research Symposium: Hypertrophic Cardiomyopathy in Cats and Humans
3/9/22—8/9/22
$5,000
Meghann Pierdon
Center for Poultry and Livestock Excellence
Assessment of welfare and behavioral impacts on commercial laying hens infested with bed bugs
3/1/22—3/1/23
$20,795
Meghann Pierdon
Pennsylvania Department of Agriculture
Origins of the piglet gut microbiome
7/1/21—6/30/22
$36,217
Dipti Pitta
Center for Poultry and Livestock Excellence
Deciphering the role of gut microbiome in reducing Haemonchus contortus infestation in PA’s small ruminant herds
11/16/21—12/1/22
$89,500
Dipti Pitta
DSM Nutritional Products AG
The effect of 3-nitrooxypropanol (3-NOP), a persistent methane inhibitor, on the ruminal microbiota in dairy cows with distinct microbial syntrophic clusters
11/24/21—12/31/23
$233,193
Laurel Redding
NIH K23
Impact of pet contact on antimicrobial-associated dysbiosis and Clostridioides difficile infection
3/9/22—2/28/27
$829,976
Antonia Rotolo (Mason Lab)
American Society of Gene and Cell Therapy
Harnessing invariant NKT cells to exploit ‘intact’ allogeneic CAR-T therapies: a pilot trial in companion dogs with spontaneous solid cancer
1/1/20—11/30/22
$50,000
Kotaro Sasaki
University of Texas at San Antonio
Advancing brain health research through male germline editing in marmosets
8/1/21—5/31/22
$42,314
Thomas Schaer
Drexel University
Orthopedic surgical devices with controlled fluid-induced expansion properties for improved bone fixation and bone integration
3/1/22—3/1/23
$99,795
Brittany Tisa
Nestlé Purina
The characteristics, motivations, and experiences of foster caregivers at animal shelters
1/1/22—12/31/23
$28,631
Natalie Toothacre (Anguera Lab)
Lupus Foundation of America, Philadelphia Tri-State Chapter
Elucidating the role of DNA methylation in dynamic X chromosome inactivation and lupus disease development in female lymphocytes
7/1/21—6/30/22
$1,000
Andrew Vaughan
ITMAT/PICAB
Modeling COVID-19 comorbidities with novel humanized ACE2 mice
3/1/22—2/28/23
$30,000
Andrew Vaughan
Moseley Foundation
Harnessing alveolar progenitor cells for lung repair after COVID-19 and beyond
9/1/21—8/31/23
$109,000
Andrew Vaughan
Monell Chemical Senses Center (sub award)
Mechanisms of inflammation-triggered taste loss and its recovery
4/1/21—3/31/22
$7,072
Zebin Xiao (Puré Lab)
Cancer Research Institute
Impact of FAP+ stromal cell depletion on the immune landscape of pancreatic ductal adenocarcinoma
1/1/22—12/31/24
$56,500


