Instruments & Applications
The Penn Vet Imaging Core (PVIC) has a variety of microscopes available to meet a broad range of imaging needs. Information on the specific instruments available in the PVIC is provided below, along with examples of some of the types of work that can be done with each of these microscopes.
Please review the services and fees that our core provides.
Scheduling Core Instruments
Registered users can access the PVIC online scheduler to reserve and use the Penn Vet Imaging Core instruments.
If you are not already registered, please email the core manager.
Instrument Details
Instrument Details
Leica Stellaris 8 FALCON Confocal/FLIM

Description
This inverted laser scanning confocal microscope has a white light laser that is tunable from 440nm to 790nm and an additional 405nm laser. Leica Lightning deconvolution is incorporated into the software. It is also a Fluorescence Lifetime Imaging Microscope (FLIM) with all the tools necessary for quantitative fluorescence lifetime measurements and analysis conveniently contained within the Leica software. A Tau-STED super-resolution module will be added to this system by the end of 2025.
Purchase of this instrument was made possible by NIH grant S10 OD032305-01A1. Please acknowledge this funding source in any publications that include work performed on the Leica Stellaris FALCON Confocal/FLIM microscope.
Technical Specifications:
Stellaris FALCON Tech Sheet (PDF)
Applications and Example
- Multiplex confocal imaging of up to at least 11 labels in a single sample
- Leica Lightning rapid deconvolution of image stacks
- Multipoint live cell confocal imaging
- Image tiling and stitching
- Fluorescence Lifetime-based Förster resonance energy transfer (FLIM-FRET)
- Differentiation of fluorophores with highly overlapping spectra by different lifetimes
- Fluorescence recovery after photobleaching (FRAP)
Leica SP8-MP Confocal/Multiphoton
Description

This upright laser scanning confocal microscope has 2-Photon capability, which provides the ability to perform intravital imaging and second harmonic generation (SHG) imaging. Intravital imaging is further facilitated by the four non-descanned hybrid detectors, resonant scanner, and special 20x 1.0 NA and 25x 0.9 NA dipping lenses on this system.
Purchase of this instrument was made possible by NIH grant S10 OD021633-01. Please acknowledge this funding source on any publications that include work performed on the Leica SP8 Multiphoton Confocal.
Technical Specifications:
SP8-Multiphoton Tech Sheet (PDF)
Leica SP8-MP External Detectors Schematic Diagram (PDF)
Applications and Example
- Intravital multiphoton imaging (isoflurane anesthesia available)
- Second harmonic generation (SHG) imaging, backward and forward
- Third harmonic generation (THG) imaging
- Image tiling with precision motorized Scientifica stage
- Fluorescence recovery after photobleaching (FRAP)
- Intensity Förster resonance energy transfer (FRET)
- Routine confocal microscopy and spectral scans
Second Harmonic Generation (SHG) imaging of collagen

The cross section of ascending aorta shown here was imaged by SHG microscopy to show fibrillar collagen (blue).
This image was taken on the Penn Vet Imaging Core’s Leica SP5 2-Photon microscope with a 20x (1.0 NA) water immersion lens. A Coherent Chameleon Ti:Sapphire laser was used to illuminate the tissue section with infrared light.
Second harmonic signal from fibrillar collagen, which is half the wavelength of the illuminating light, was then collected into non-descanned detector NDD1.
Additional images of tissue autofluorescence were simultaneously acquired in wavelengths within the ranges of 495-560 and 560-619 (NDD2 and NDD3; pseudocolored green and red, respectively). Autofluorescence was present in all three channels and combined to form a grayish color in the image overlay shown here, differentiating the SHG signal (present only in the blue channel) from autofluorescence.
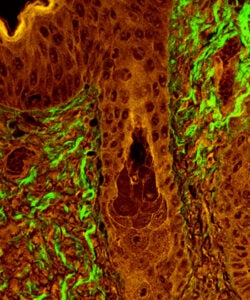
For this image, SHG microscopy was used to visualize fibrillar collagen (green) in a section of skin at the epidermal and dermal junction, with a hair follicle visible in the middle of the field.
This image was taken on the Penn Vet Imaging Core’s Leica SP5 2-Photon microscope with a 20x (1.0 NA) water immersion lens. Fibrillar collagen present in the section combined 900 nm wavelength photons from a Coherent Chameleon Ti:Sapphire laser into light of 450 nm wavelength, which was then collected into non-descanned detector NDD1.
An additional image of tissue autofluorescence was simultaneously acquired for wavelengths within the range 495-560 nm (NDD2; pseudocolored red). In the overlay shown here, autofluorescence collected by both detectors combined to form an orange color, whereas SHG signal was only detected by NDD1 and therefore appears as green in the image overlay.
Image courtesy of Ashley Case, Becky Brisson, and Susan Volk, Dept. of Clinical Studies, UPenn School of Veterinary Medicine.
Confocal Microscopy and 3D reconstruction of Z-stacks
This movie shows a 3D reconstruction of scanning laser confocal images of a hydrogel (red) with silica particles (green).
The images were acquired on the Penn Vet Imaging Core’s Leica SP5 upright confocal/multiphoton microscope with a 20x (1.0 NA) water immersion lens. The 488 nm Argon laser and 543 nm HeNe laser were used to illuminate the sample and the fluorescence emitted was collected with the internal spectral detectors.
The image Z-stack was rendered into the 3D image shown here with Volocity software from Perkin Elmer, accessed through the core’s Volocity license server.
Image courtesy of Gaoxiang Wu and Shu Yang, UPenn School of Engineering and Applied Science.
Leica DM6000B Upright Widefield Fluorescence Microscope
This upright widefield microscope is equipped with both color and monochrome cameras, making it suitable for imaging either histology slides or fluorescently labeled samples.
Technical Specifications:
Leica DM6000B Widefield tech sheet (PDF)
Applications and Examples
- Acquisition of full-color images from histology slides
- Acquisition of fluorescence images (up to 4 colors)
- Automated tile and stitch acquisition of large areas
- Motorized Z-stack acquisition for deconvolution with Huygens software
- Differential interference contrast images with 40x, 63x, and 100x
Fluorescence microscopy
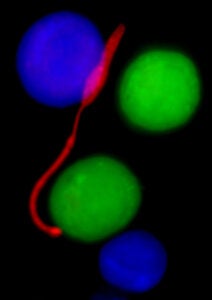
The parasite Leishmania major (promastigote stage; red) is shown here among alternatively activated (M2) macrophages (green) and resident peritoneal macrophages (blue).
These images were taken on the Penn Vet Imaging Core’s Nikon E600 fluorescence microscope with a 100x oil immersion lens.
Images are courtesy of Tiffany Weinkopff and Phillip Scott, Dept. of Pathobiology, UPenn School of Veterinary Medicine.
Transmitted light microscopy
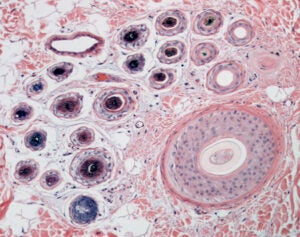
Cross sections of a primary (larger) and multiple secondary (smaller) hair follicles characteristic of compound hair follicles in dogs and cats. This image was taken on the Penn Vet Imaging Core’s Nikon E600 microscope with a 10x objective lens.
Sample prepared by the Penn Vet Diagnostic Lab, photo by Gordon Ruthel, description provided by Charles Bradley.
Transmitted light microscopy
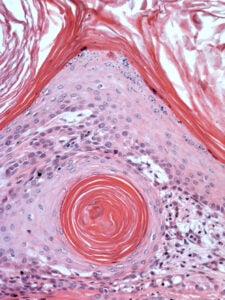
A keratin-filled cyst at the periphery of an infundibular keratinizing acanthoma, a common benign follicular neoplasm in dogs. This image was taken on the Penn Vet Imaging Core’s Nikon E600 microscope with a 20x objective lens.
Sample prepared by the Penn Vet Diagnostic Lab, photo by Gordon Ruthel, description provided by Charles Bradley.
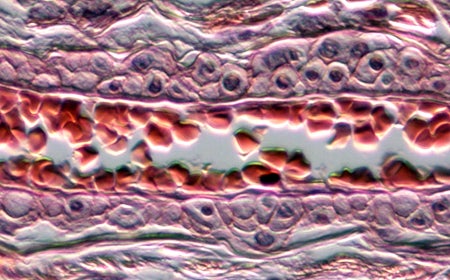
Transmitted light microscopy
Erythrocytes within a meningeal arteriole of a dog brain. This image was taken on the Penn Vet Imaging Core’s Nikon E600 microscope with a 40x objective lens and DIC optics.
Sample prepared by the Penn Vet Diagnostic Lab, photo by Gordon Ruthel, description provided by Charles Bradley.
Leica DMI4000 with Yokagawa CSU-X1 Spinning Disk Confocal Attachment
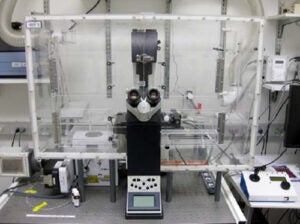
The Leica DMI4000 Microscope with Yokagawa CSU-X1 Spinning Disk Confocal Attachment offers a full environmental enclosure, programmable motorized stage, and sensitive EM-CCD camera make this spinning disk confocal system ideal for long-term multipoint timelapse microscopy. It is also excellent for large-scale automated tile acquisition and image stitching.
Technical Specifications:
Spinning Disk Tech Sheet (PDF)
Purchase of this instrument was made possible by NIH grant S10 RR027128-01. Please acknowledge this funding source on any publications that include work performed on the Spinning Disk Confocal.
Applications and Examples
- Long-term multi-point timelapse microscopy
- Fluorescence, brightfield, differential interference contrast (DIC), and polarization microscopy
- Large-scale image tile acquisition and stitching
Multipoint Timelapse on Spinning Disk
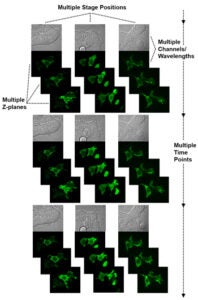
A timelapse experiment done on the Penn Vet Imaging Core’s Yokagawa spinning disk confocal microscope is shown schematically. Multiple stage positions, different imaging modes (such as brightfield and laser confocal of GFP fluorescence in this example), number and range of depth for multiple focal planes, and time interval and duration can all be specified within the MetaMorph software to automate image acquisition. A full environmental enclosure around the microscope allows cells to be maintained at a stable 37°C temperature and 5% CO2 level for long-term imaging.
Images of HEK293T cells transfected to express GFP-tagged ebola VP40 protein courtesy of Gordon Ruthel, Xiaohong Liu, Bruce Freedman, and Ron Harty, Department of Pathobiology, UPenn School of Veterinary Medicine.
VP-40 Timelapse Movie
This timelapse movie of HEK293T cells that were transfected to express a GFP-tagged ebola VP40 protein was acquired on the Penn Vet Imaging Core’s Yokagawa spinning disk confocal microscope. The images were rendered as color heatmaps of GFP fluorescence intensity with MetaMorph imaging software. Elapsed time is shown as hours:minutes:seconds in the lower left corner.
Movie courtesy of Gordon Ruthel, Xiaohong Liu, Bruce Freedman, and Ron Harty, Department of Pathobiology, UPenn School of Veterinary Medicine.
Large-scale image tile acquisition and stitching
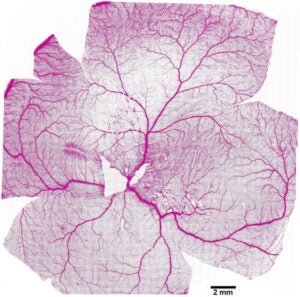
The vasculature pattern of a dog retina pictured here was pieced together from 1600 individual image fields acquired with a 10x objective lens and 488 nm laser on the Penn Vet Imaging Core’s Yokagawa spinning disk confocal microscope.
For each field, an image Z-stack was taken at 5 µm intervals over an almost 100 µm thickness and the maximal projection image for each field was used for the montage.
The images were stitched together with MetaMorph imaging software, which was also used to automate the acquisition of the 1600 individual images. The resulting fluorescence image was color-inverted for the view presented here.
Image courtesy of William Beltran, Section of Ophthalmology, Department of Clinical Studies, UPenn School of Veterinary Medicine.
ImageXpress Micro 4 High Content

The ImageXpress is a high content imaging device for automated imaging and analysis of multiwell culture plates. It is capable of acquiring fluorescence and transmitted light images. This system has integrated environmental controls and laser-based autofocusing for long-term imaging of live cells in addition to endpoint assays.
Technical Specifications:
ImageXpress Micro 4 High Content Imaging Device (PDF)
Applications
- A wide variety of multi-well culture plate cell-based fluorescence endpoint assays
- Multi-well culture plate live cell kinetic assays (fluorescence and/or phase contrast)
GE DeltaVision OMX Structured Illumination Super-Resolution Microscope

This microscope provides super-resolution images by means of structured illumination microscopy (SIM). It is capable of both 2D-SIM and 3D-SIM, as well as 2D-SIM in combination with TIRF (total internal reflection fluorescence) microscopy. This system has integrated environmental controls and laser-based autofocusing for long-term imaging of live cells.
Technical Specifications:
GE DeltaVision OMX Tech Sheet (PDF)
Applications and Examples
- 2D- or 3D-SIM super-resolution imaging of fluorescently labeled fine structures (i.e. approx. 100 nm in size)
- 2D-SIM TIRF of live cells for super-resolution imaging of structures at or near the cell membrane close to the coverslip
- Long-term live cell imaging by conventional widefield epifluorescence, SIM, or SIM TIRF
SIM TIRF Microscopy
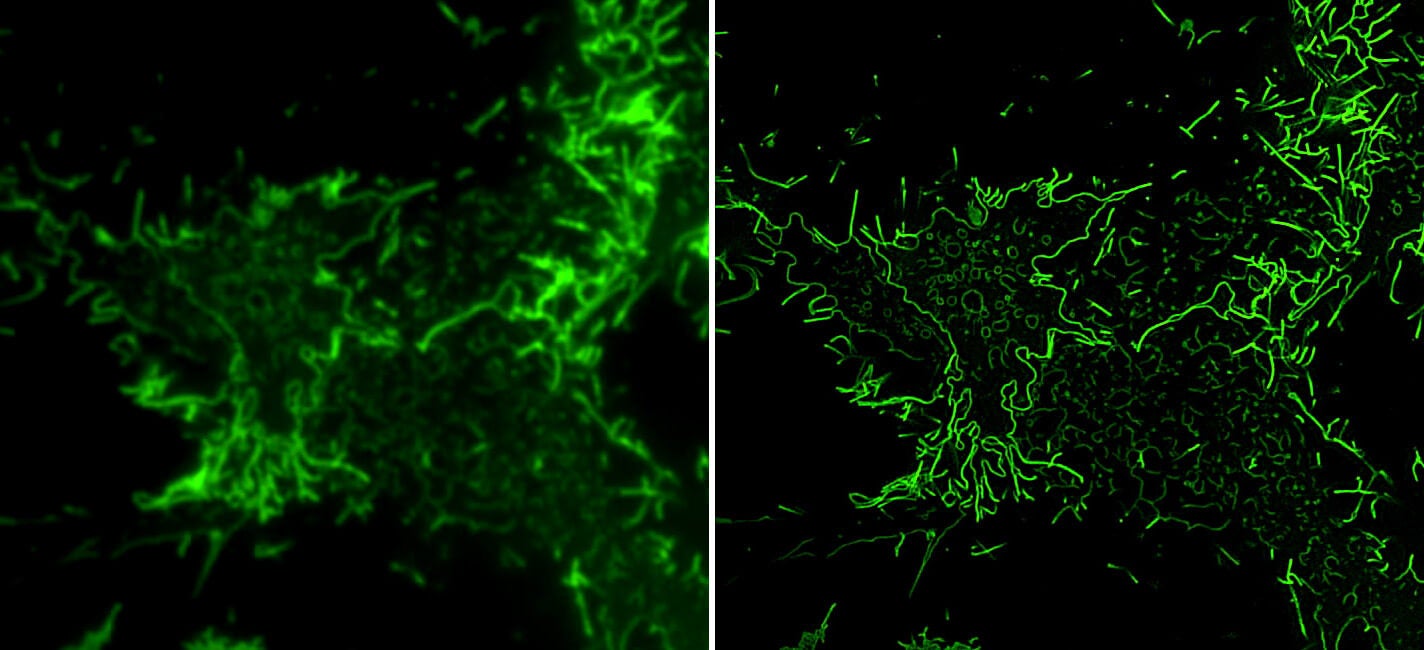
The images and video (below) show HEK-293T cell transfected to express GFP-tagged ebola VP40 protein imaged using SIM TIRF (Structured Illumination Microscopy and Total Internal Reflection Fluorescence) on the DeltaVision OMX instrument. Ebola VP40 protein is able to assemble into virus-like particles (VLPs) that are able to bud from the cell membrane and are used as a noninfectious model system to study processes involved in virus budding. Although VLPs can be microns long, they are only about 80 nm in width. The images show TIRF images without (left) and with (right) SIM super-resolution for comparison. The video is a time-lapse sequence with an image acquired every 5 seconds.
Time-lapse video of Ebola VP40 protein using the GE DeltaVision OMX
Ebola VP40 protein is able to assemble into virus-like particles (VLPs) that are able to bud from the cell membrane and are used as a noninfectious model system to study processes involved in virus budding. Although VLPs can be microns long, they are only about 80 nm in width. In this time-lapse sequence, an image is acquired every 5 seconds.
Images courtesy of Xiaohong Liu and Bruce Freedman, UPenn School of Veterinary Medicine.
Leica SP5-II Confocal/Fluorescence
Lifetime Imaging Microscope

This is a legacy instrument that is still functional but is no longer supported by Leica. Please note that the FLIM functionality on this instrument has been retired, but it can still be used as a standard confocal microscope, now offered at a discounted rate. For FLIM experiments, please use the Leica Stellaris FALCON confocal/FLIM.
The fully integrated PicoQuant fluorescence lifetime imaging system on this inverted laser scanning confocal microscope gives users the option of analyzing fluorescent probe distribution based on fluorescence lifetime in addition to fluorescence intensity. This is particularly useful for examining dynamic protein-protein interactions by Förster resonance energy transfer (FRET), which can be measured as a shortening in the average fluorescence lifetime of the FRET donor.
Purchase of this instrument was made possible by NIH grant S10 RR027128-01. Please acknowledge this funding source on any publications that include work performed on the Leica SP5 FLIM Confocal.
Technical Specifications:
Leica SP5 FLIM tech sheet (PDF)
Applications and Examples
- Routine confocal microscopy and spectral scans
- FLIM-based Förster resonance energy transfer (FRET)
Confocal Microscopy and 3D reconstruction of Z-stacks
This movie shows a 3D reconstruction of scanning laser confocal images of GFP-tagged Ebola VP40 protein (green) being expressed by an HEK293T cell. The cells were fixed then stained with HCS CellMask Deep Red (red).
Images were taken on the Penn Vet Imaging Core’s Leica SP5 II inverted confocal/FLIM microscope with a 100x oil immersion lens by scanning the sample with 488 nm Argon and 647 nm HeNe confocal lasers. Image Z-stacks were rendered into the 3D image shown here with Volocity software from Perkin Elmer, accessed through the core’s Volocity license server.
Image courtesy of Jonathan Madara, Gordon Ruthel, Bruce Freedman, and Ron Harty, Department of Pathobiology, UPenn School of Veterinary Medicine.
FLIM-based Förster Resonance Energy Transfer (FRET)
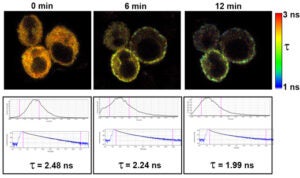
The fluorescence lifetime of Cerulean fluorescent protein was measured at various times after treatment with thapsigargin in Jurkat cells transfected to express Cerulean-STIM1 and Venus-Orai1. Thapsigargin causes a depletion of ER Ca2+ stores, resulting in STIM1 relocalization to associate with Orai1 on the plasma membrane and form CRAC Ca2+ channels.
As Cerulean-STIM1 associates with Venus-Orai1, FRET occurs between Cerulean (the donor) and Venus (the acceptor), which results in a shortening in the average fluorescence lifetime of Cerulean.
The images are fluorescence lifetime heatmaps based on the scale shown to the right. These images correspond to graphs of average lifetime, decay curves, and average Cerulean fluorescence lifetimes (τ).
Fluorescence lifetime imaging was performed on the Penn Vet Imaging Core’s Leica SP5 II inverted confocal/FLIM microscope with a 63x water immersion lens by scanning the sample with a 405 nm pulsed diode laser.
Images courtesy of Xiaohong Liu, Gordon Ruthel, and Bruce Freedman, Department of Pathobiology, UPenn School of Veterinary Medicine.
Leica Total Internal Reflection Fluorescence (TIRF) Microscope

This inverted microscope has filter wheels and software specifically for Fura-2 ratio calcium imaging. It also has separate TIRF functionality: Through evanescent wave illumination that can be adjusted to penetrate as little as 70 nm, events at or near the cell membrane can be imaged with high Z-Axis resolution.
This microscope is available by special arrangement with the laboratory of Dr. Bruce Freedman. Please contact Dr. Freedman or Gordon Ruthel, the core manager, directly to schedule use of this microscope.
Applications and Examples
- Fura-2 ratio calcium imaging
- Total Internal Reflection Fluorescence (TIRF) microscopy for visualizing events at or near the cell membrane
Leica Total Internal Reflection Fluorescence (TIRF) Microscope
This movie shows a TIRF microscopy timelapse series of GFP-tagged ebola VP40 protein (green) produced by a transfected HEK293T cell. Images were taken on a Leica TIRF microscope with a 100x oil immersion lens. Evanescent wave excitation from a 488 nm laser was set to a penetration depth of 70 nm, such that only events at or near the cell/coverglass interface were visualized. The TIRF microscope is available to Penn Vet Imaging Core users by special arrangement with Dr. Bruce Freedman.
Movie courtesy of Jonathan Madara, Gordon Ruthel, Ron Harty, and Bruce Freedman, Department of Pathobiology, UPenn School of Veterinary Medicine.
Nikon SMZ 1000 Stereo Microscope
This stereomicroscope is provided primarily for use in microdissection. Although it does not currently have a dedicated camera associated with it, the core manager can temporarily attach the color camera from the Leica DM6000 widefield microscope for users who require photographs.
Technical Specifications:
Nikon SMZ 1000 stereo microscope tech sheet (PDF)
Applications and Examples
Anopheles gambiae mosquitoes
- A male and female are shown on the left.
- A higher magnification image of a mosquito head is shown on the right.
These images were taken with a Nikon Digital Sight DS-Fi1 color camera attached to the Penn Vet Imaging Core’s Nikon SMZ 1000 stereo microscope. The mosquitoes were illuminated from above with a ring light attachment.
Images are courtesy of Dr. Michael Povelones, Penn Vet Department of Pathobiology.


