Wang Laboratory
About Us
The Wang Laboratory focuses on the study of regulation of meiosis, the biology of small non-coding RNAs (piRNAs), epigenetics of transposable elements, and biology of spermatogonial stem cells.
Meiosis, a cell division unique to germ cells, allows the reciprocal exchange of genetic material between paternal and maternal genomes. Meiosis generates the genetic diversity necessary for evolution of species.
Abnormality in meiosis is a leading cause of birth defects and infertility. Our research interests include molecular genetics of chromosomal synapsis, DNA double-strand break repair, homologous recombination, genetic causes of male infertility in humans, piRNA biogenesis, and epigenetic silencing of transposable elements.
At the Wang laboratory, we have performed two genome-wide screens to identify novel factors that regulate germ cell development in mice: a genomics screen has identified 36 germ cell-specific genes; a proteomics screen has uncovered more than 50 meiotic chromatin-associated proteins.
Functional characterization of a number of new genes in the Wang Laboratory has uncovered novel regulatory mechanisms underlying key biological processes unique to germ cells. On one hand, our studies provide molecular insights into the development of germ cells in mice. On the other hand, these mouse studies have important implications for understanding the genetic causes of male infertility in humans.
We employ a battery of the state-of-the-art technologies in our research: gene targeting, genome editing, genomics, proteomics, cell biological and molecular biological approaches.
Our Research
Self-renewal of spermatogonial stem cells is vital to lifelong production of male gametes and thus fertility. However, the underlying mechanisms remain enigmatic. We have discovered that DOT1L, the sole H3K79 methyltransferase, is required for spermatogonial stem cell self-renewal. Mice lacking DOT1L fail to maintain spermatogonial stem cells, characterized by a sequential loss of germ cells from spermatogonia to spermatids and ultimately a Sertoli cell only syndrome. Inhibition of DOT1L reduces the stem cell activity after transplantation. DOT1L promotes expression of the fate-determining HoxC transcription factors in spermatogonial stem cells. Furthermore, H3K79me2 accumulates at HoxC9 and HoxC10 genes. Our findings identify an essential function for DOT1L in adult stem cells and provide an epigenetic paradigm for regulation of spermatogonial stem cells.
Piwi-interacting RNAs are a diverse class of small non-coding RNAs implicated in the silencing of transposable elements and the safeguarding of genome integrity. In mammals, male germ cells express two genetically and developmentally distinct populations of piRNAs at the pre-pachytene and pachytene stages of meiosis, respectively.
Pre-pachytene piRNAs are mostly derived from retrotransposons and required for their silencing. In contrast, pachytene piRNAs originate from ~100 genomic clusters depleted of transposable elements. We find that MOV10L1, a 5′ — 3′ RNA helicase, is a master regulator of biogenesis of all piRNAs. Mov10l1 is one of the 36 germ cell-specific genes identified in our genomic screen. Mechanistically, MOV10L1 binds to piRNA precursors to initiate piRNA biogenesis. MOV10L1 interacts with PIWI proteins and TDRD1 and its RNA helicase activity is required to resolve the thermostable G-quadruplex secondary structures in piRNA precursors.
DNA methylation is a major silencing mechanism of transposable elements (TEs). We report that TEX15, a testis-specific protein that we previously identified, is required for TE silencing. TEX15 is expressed in embryonic germ cells and functions during genome-wide epigenetic reprogramming. The Tex15 mutant exhibits DNA hypomethylation in TEs at a level similar to Mili and Dnmt3c but not Miwi2 mutants. TEX15 is associated with MILI in testis. As loss of Tex15 causes TE desilencing with intact piRNA production, our results identify TEX15 as a new essential epigenetic regulator that may function as a nuclear effector of MILI to silence TEs by DNA methylation.
We have identified TEX11 as the first X chromosome-encoded meiosis-specific factor in mammals. In principle, meiosis-specific genes could be located anywhere in the genome. However, no mouse sex chromosome-linked mutants with meiosis-specific defects had been reported, leading to the perception that meiosis-specific factors are rarely if ever encoded by the sex chromosomes. We were the first to clone Tex11, an X-linked germ cell-specific gene, in our genomic screen.
In a recent study, by ablating the function of Tex11 in mice, we have demonstrated that Tex11 is essential for meiosis and fertility in males. Our findings have important implications for male infertility in humans, which accounts for about half of the cases of infertility among couples. An estimated 15% of couples are affected by infertility worldwide. Given that disruption of Tex11 causes azoospermia in mice, we surmise that mutations in the human TEX11 gene could be found in infertile men.
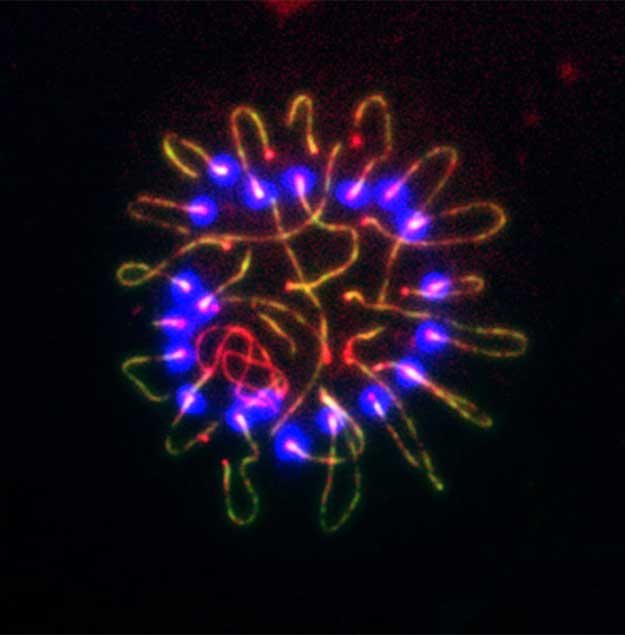
During meiosis, homologous chromosomes undergo synapsis and recombination. Meiotic recombination enables the reciprocal exchange of genetic material between parental homologous chromosomes, and ensures faithful chromosome segregation during meiosis in sexually reproducing organisms.
This process relies on the complex interaction of DNA repair factors and many steps remain poorly understood in mammals. The arrangement of homologous chromosomes is tightly regulated by the synaptonemal complex (SC). SYCP2 is an integral component of SCs in mammals. Our genetic and cell biological studies demonstrate that SYCP2 is required for the formation of SCs and chromosomal synapsis.
We also find that TEX11 interacts with SYCP2 and is a novel constituent of meiotic nodules involved in recombination. TEX11 promotes both synapsis and recombination, and thus may provide a physical link between these two fundamental meiotic processes.
We recently identified MEIOB, a meiosis-specific protein, in a proteomics screen for novel meiotic chromatin-associated proteins in mice. MEIOB contains an OB domain with homology to one of the RPA1 OB folds and thus is a meiosis paralogue of RPA1, a ubiquitously expressed single-strand DNA-binding protein. MEIOB binds to single-stranded DNA and exhibits 3′-5′ exonuclease activity. MEIOB forms a complex with RPA and with SPATA22, another meiosis-specific protein in vertebrates. These three proteins co-localize in foci that are associated with meiotic chromosomes. Strikingly, chromatin localization and stability of MEIOB depends on SPATA22 and vice versa. Meiob-null mouse mutants exhibit a failure in meiosis and sterility in both sexes. Our data suggest that MEIOB may be required for the second end capture during meiotic recombination in mammals.
The meiotic prophase I to metaphase I (PI/MI; equivalent to the mitotic G2/M transition) transition requires chromosome desynapsis and metaphase competence acquisition. However, control of these major meiotic events is poorly understood. We identify an essential role for SKP1, a core subunit of the SKP1-Cullin-F-box (SCF) ubiquitin E3 ligase, in the PI/MI transition. SKP1 localizes to synapsed chromosome axes and evicts HORMAD proteins from these regions in meiotic spermatocytes. SKP1-deficient spermatocytes display premature desynapsis, precocious pachytene exit, loss of PLK1 and BUB1 at centromeres, but persistence of HORMAD, γH2AX, RPA2, and MLH1 in diplonema. Strikingly, SKP1-deficient spermatocytes show sharply reduced MPF activity and fail to enter MI despite treatment with okadaic acid. SKP1-deficient oocytes exhibit desynapsis, chromosome misalignment, and progressive postnatal loss. Therefore, SKP1 maintains synapsis in meiosis of both sexes. Furthermore, our results support a model where SKP1 functions as the long-sought intrinsic metaphase competence factor to orchestrate MI entry during male meiosis.
Publications
TRIP13 localizes to synapsed chromosomes and functions as a dosage-sensitive regulator of meiosis. Chotiner JY, Leu NA, Yang F, Cossu IG, Guan Y, Lin H, and Wang PJ. eLife. 2024.
The N-terminal modification of HORMAD2 causes its ectopic persistence on synapsed chromosomes without meiotic blockade. Cossu IG, Leu NA, Guan Y, and Wang PJ. Reproduction. 2024 Feb 1:REP-23-0330.
ARF6, a component of intercellular bridges, is essential for spermatogenesis in mice. Wong H, Chen T, Wang PJ, Holzman L. Dev. Biology. 2024 Jan 17:508:46-63.
The MOV10 RNA helicase is a dosage-dependent host restriction factor for LINE1 retrotransposition in mice. Guan Y, Gao H, Leu NA, Vourekas A, Alexiou P, Maragkakis M, Mourelatos Z, Kang Z, Liang G, and Wang PJ. PLoS Genetics. 2023;19(5):e1010566.
The DOT1L-MLLT10 complex regulates male fertility and promotes histone removal during spermiogenesis. Development. Lin L, Cossu IG, Leu NA, Deshpande AJ, Bernt KM, Luo M, & Wang PJ. 2023;150(9):dev201501.
The germ cell-specific RNA binding protein RBM46 is essential for spermatogonial differentiation in mice. PLoS Genetics. Peart NJ, Johnson TA, Lee S, Sears MJ, Yang F, Quesnel-Vallières M, Feng H, Recinos Y, Barash Y, Zhang C, Hermann BP, Wang PJ, Geyer CB, Carstens RP. 2022;18(9):e1010416. https://doi:10.1371/journal.pgen.1010416.
Histone methyltransferase DOT1L is essential for self-renewal of germline stem cells. Lin H, Cheng K, Kubota H, Lan Y, Riedel SS, Kakiuchi K, Sasaki K, Bernt KM, Bartolomei MS, Luo M, Wang PJ. Genes & Development. 2022;36(11-12):752-763. https://doi: 10.1101/gad.349550.122.
Genetic characterization of a missense mutation in the X-linked TAF7L gene identified in an oligospermic man. Ling L, Li F, Yang P, Oates RD, Silber S, Kurischko C, Luca FC, Leu NA, Zhang J, Yue Q, Skaletsky H, Brown LG, Rozen S, Page DC, Wang PJ, and Zheng K. Biology of Reproduction. 2022;107(1):157-167 . https://academic.oup.com/biolreprod/article/107/1/157/6583998
SCF ubiquitin E3 ligase regulates meiotic DNA double-strand breaks in early meiotic recombination. Guan Y, Lin H, Leu NA, Ruthel G, Fuchs SY, Busino L, Luo M, and Wang PJ. Nucleic Acids Research, 2022 50(9):5129-5144. https://academic.oup.com/nar/article/50/9/5129/6576356
C2CD6 regulates targeting and organization of the CatSper calcium channel complex in sperm flagella. Development. Yang F, Gervasi MG, Leu NA, Orta G, Tourzani DA, De la Vega-Beltrán JL, Ruthel G, Darszon A, Visconti PE, Wang PJ. 2022;149(2):dev199988. https://journals.biologists.com/dev/article/149/2/dev199988/273995/C2CD6-regulates-targeting-and-organization-of-the
Recurrent pregnancy loss in mice lacking the X-linked Ccnb3 gene. Biology of Reproduction. Chotiner JY, Leu NA, Yang X, and Wang PJ. 2022;106(3):382-384. https://academic.oup.com/biolreprod/article/106/3/382/6446354.
YTHDC2 is essential for pachytene progression and prevents aberrant microtubule-driven telomere clustering in male meiosis. Liu R, Kasowitz SD, Homolka D, Leu NA, Shaked JT, Ruthel G, Jain D, Keeny S, Luo M, Pillai RS, and Wang PJ. Cell Reports. 2021;37(11):110110. https://doi.org/10.1016/j.celrep.2021.110110
HDAC3 controls male fertility through enzyme-independent transcriptional regulation at the meiotic exit of spermatogenesis. Yin H, Kang Z, Zhang Y, Gong Y, Liu M, Xue Y, He W, Wang Y, Zhang S, Xu Q, Fu K, Zheng B, Jie Xie J, Zhang J, Wang Y, Lin M, Zhang Y, Feng H, Xin C, Guan Y, Huang C, Guo X, Wang PJ, Baur JA, Zheng K, Sun Z, Ye L. Nucleic Acids Res. 2021;49(9):5106-5123.
Golden opportunity for piRNA in female fertility. Guan, Y and Wang, PJ. Nature Cell Biology. 2021; 23(9):936-938. https://doi.org/10.1038/s41556-021-00749-z.
yama, a mutant allele of Mov10l1, disrupts retrotransposon silencing and piRNA biogenesis. Guan Y, Keeney S, Jain D, and Wang PJ. PLoS Genetics. 2021; 17(2):e1009265. https://doi.org/10.1101/2020.11.26.399659.

Director, wang Laboratory
P. Jeremy Wang, MD, PhD
Professor of Developmental Biology, Department of Biomedical Sciences, School of Veterinary Medicine University of Pennsylvania
Find Us
University of Pennsylvania School of Veterinary Medicine
390EC Rosenthal Building
3800 Spruce Street
Philadelphia, PA 19104-453
Our Team

Ankit Jaiswal

Zhenlong Kang

Maya Kennedy

Cong Liu

Fang Yang, MD

Yiyun Zhang

Penn Vet and Penn Medicine Researchers Receive Nearly $6 Million in Renewed NIH Funding to Study Epigenetics of Reproduction in Animals and Humans
A multidisciplinary group of researchers from the University of Pennsylvania’s School of Veterinary Medicine (Penn Vet) and the University’s Perelman School of Medicine (Perelman) have received $5.95 million in renewed…
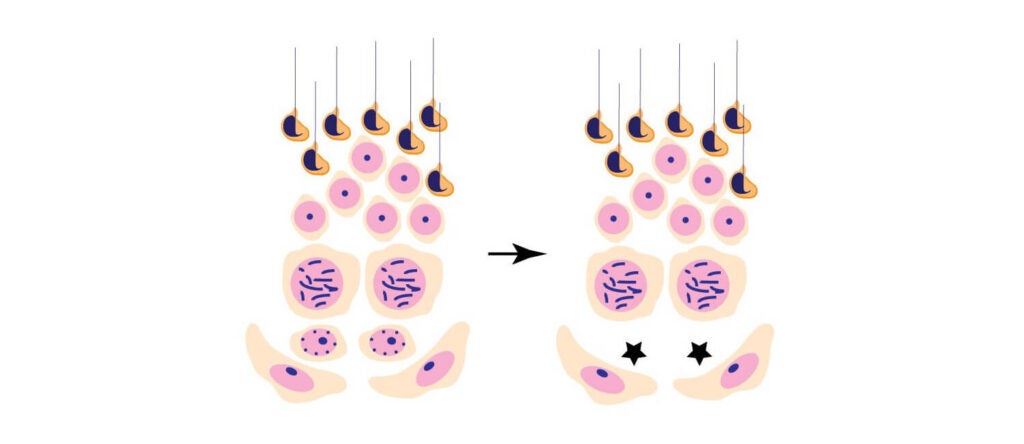
A newly identified stem cell regulator enables lifelong sperm production (link is external)
Research led by Dr. Jeremy Wang has discovered that the enzyme DOT1L, a stem cell renewal factor, is essential for mice to produce sperm throughout their adult lives.

P. Jeremy Wang, MD, PhD, Named Ralph L. Brinster President’s Distinguished Professor at Penn Vet
The awarding of a named, endowed professorship is the highest honor bestowed upon a faculty member at the University of Pennsylvania and reflects a commitment to scientific discovery, mentorship, and…
