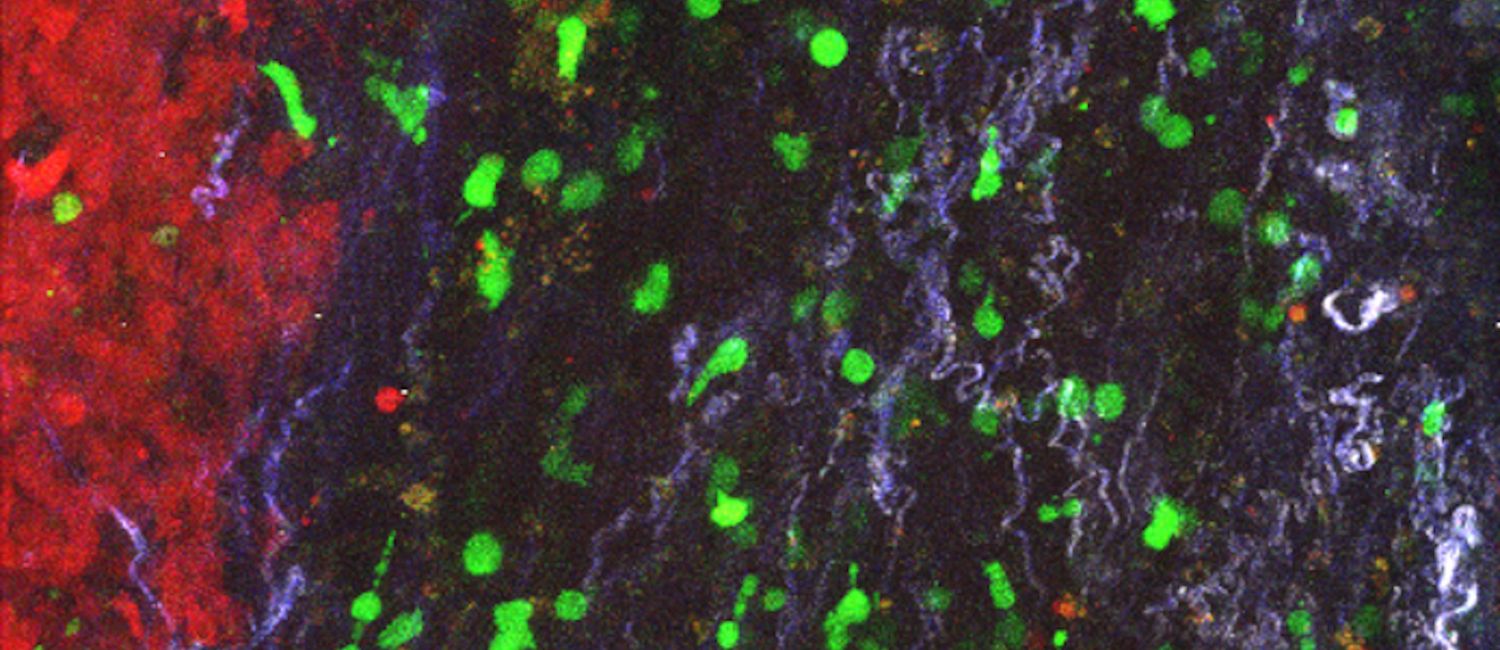
 Stroma-targeting CAR T cells (green) accumulate in the stroma surrounding a tumor in a mouse model of pancreatic cancer. (Image: Zebin Xiao)
Stroma-targeting CAR T cells (green) accumulate in the stroma surrounding a tumor in a mouse model of pancreatic cancer. (Image: Zebin Xiao)
While immunotherapies have shown great promise in treating blood cancers, most clinical trials aimed at treating solid tumors such as pancreatic or lung cancer have failed. Researchers have long thought that solid tumors’ resistance to treatment is due to the tumor microenvironment—the cells and matrix that surround solid tumors—but the exact mechanisms behind this blockade were unclear, until now.
In a new study, University of Pennsylvania researchers reveal how the tumor microenvironment prevents T cells from attacking tumors. Using mouse models, they showed that cancer-associated fibroblasts along with extracellular matrix within the tumor microenvironment create a physical barrier to T cell entry, and these cells also actively suppress T cell function. When the researchers used CAR T cells to target and remove these fibroblasts, rather than targeting the tumor cells themselves, T cells were able to infiltrate and attack the tumor.
“The physical barrier and immunosuppressive environment derived from cancer-associated fibroblasts limits or traps T cells and prevents them from entering into the tumor,” says first author Zebin Xiao, a physician and postdoctoral researcher in the School of Veterinary Medicine. “We showed that targeting those fibroblasts can disrupt that barrier and has a very great tumor inhibition effect.”
The study, which was a collaboration between researchers in the School of Veterinary Medicine, Perelman School of Medicine, and the School of Arts & Sciences, was published in the journal Nature Communications.
The researchers say that using a dual approach—by first targeting cancer-associated fibroblasts and then sending in tumor-targeting CAR T cells—could be a therapeutic breakthrough for treatment-resistant solid tumors. “It's sort of like sending in a lead team to blow up things in the way,” says senior author Ellen Puré.
Solid tumors are surrounded by a matrix of cells and fibrous connective tissue called “stroma”. Previous studies have shown that stroma plays a role in preventing T cells from attacking solid tumors, but it was unclear how. The importance of stroma also seems to vary between cancer types, and is particularly striking in pancreatic ductal adenocarcinoma, a type of pancreatic cancer in which stroma can represent more than 90% of the tumor volume.
Understanding the stroma’s role in blocking anti-tumor immune function is an essential step towards to overcoming this hurdle. To try to pinpoint the mechanisms involved, the team engineered CAR T cells to zero-in and remove cancer-associated fibroblasts, cells within stroma that produce the connective tissue matrix. Specifically, they focused on a subset of cancer-associated fibroblasts that produce an enzyme—fibroblast activation protein (FAP)—that regulates fibroblast growth. Then, they used live imaging to observe interactions between the CAR T cells, the stroma, and the tumor cells within a mouse model of pancreatic cancer.
The imaging technique that was perfected by Xiao was crucial in enabling the researchers to identify mechanisms behind stroma’s barrier function.
They found that removing these cancer-associated fibroblasts effectively broke down the physical barrier surrounding the pancreatic cancer cells so that T cells could infiltrate the tumor. The treatment also disrupted the immunosuppressive environment so that, once they had infiltrated, T cells and other immune cells could effectively attack the tumor cells.
“This is the first proof that these stromal cells play a critical role in both forming a physical barrier so that T cells can’t get in, and also in creating that immunosuppressive environment,” Puré says, “And we could just reverse all of that.”
In some experiments, after treating the solid tumors in the mice with fibroblast-targeting CAR T cells, the researchers followed-up by treating the tumors with another set of CAR T cells that had been engineered to target a tumor antigen called mesothelin. Though mesothelin-targeting CAR T cells are effective at targeting cancer cells in vitro, previous clinical trials in humans have shown that they are ineffective at treating solid tumors in patients. The researchers wanted to see whether removing the stroma would allow these T cells to perform, and they showed that, indeed, it did. When administered after treatment with stroma-targeting CAR T cells, mesothelin-targeting CAR T cells were able to enter the tumor and carry out their function. This dual approach resulted in tumor regression and prevented metastasis.
Though the study’s main focus was pancreatic cancer, the researchers also successfully tested the method on several other types of solid tumor including lung cancer and mesothelioma. “It probably has implications for a fairly broad range of solid tumors,” Puré says.
However, the strongest effects so far were seen for pancreatic cancer. “Disrupting the stroma had the greatest impact in pancreatic cancer, because the more stroma, the more the tumor is dependent on it, and so the more it's susceptible to this treatment,” Puré says.
Now, the team is working towards translating this method into both human and veterinary clinics. “We're inching towards the clinic,” Puré says. “It's a broad program involving quite a few people here at Penn.”
Zebin Xiao is a postdoctoral researcher in the School of Veterinary Medicine at the University of Pennsylvania.
Ellen Puré is Grace Lansing Lambert Professor of Biomedical Science in the School of Veterinary Medicine and director of the Penn Vet Cancer Center.
In addition to Puré and Zhao, the study authors were: Penn Vet’s Leslie Todd, Li Huang, Zhen Lu, Meghan Kopp, Yue Li, Nimisha Pattada, and Charles-Antoine Assenmacher; the Perelman School of Medicine’s Estela Noguera-Ortega, Lili Huang, John Scholler, Carl H. June, and Steven M. Albelda; and the School of Arts & Sciences’ Wenqun Zhong and Wei Guo.
This work was supported by TMUNITY Therapeutics, NIH/NCI P01, Cancer Research Institute and CRI Irvington fellowship Grant#: CRI4168. The veterinary pathologists performing the histopathological analysis are part of the University of Pennsylvania Penn Vet Comparative Pathology Core Facility (RRID:SCR_022438) and are supported by the Abramson Cancer Center Support Grant (P30 CA016520).