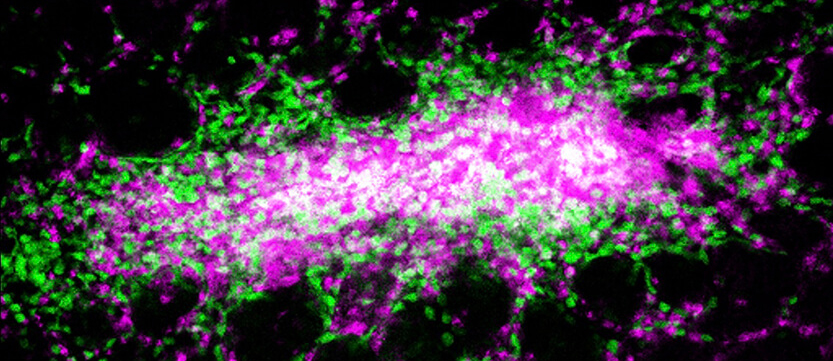
 Researchers from Penn Vet observed, for the first time, an intestinal pyogranuloma, formed in response to Yersinia infection. The organized grouping of cells includes monocytes (in green), neutrophils (in magenta), and Yersinia bacteria (in white), and depends on monocytes to form and to control infection, the team found. (Image: Courtesy of the Brodsky Laboratory)
Researchers from Penn Vet observed, for the first time, an intestinal pyogranuloma, formed in response to Yersinia infection. The organized grouping of cells includes monocytes (in green), neutrophils (in magenta), and Yersinia bacteria (in white), and depends on monocytes to form and to control infection, the team found. (Image: Courtesy of the Brodsky Laboratory)
Yersinia bacteria cause a variety of human and animal diseases, the most notorious being the plague, caused by Yersinia pestis. A relative, Yersinia pseudotuberculosis, causes gastrointestinal illness and is less deadly, but naturally infects both mice and humans, making it a useful model for studying its interactions with the immune system.
These two pathogens, as well as a third close cousin, Y. enterocolitica, which affects swine and can cause food-borne illness if people consume infected meat, have many traits in common, particularly their knack for interfering with the immune system’s ability to respond to infection.
The plague pathogen is blood-borne and transmitted by infected fleas. Infection with the other two depends on ingestion. Yet the focus of much of the work in the field had been on interactions of Yersinia with lymphoid tissues, rather than the intestine. A new study of Y. pseudotuberculosis led by a team from Penn’s School of Veterinary Medicine and published in Nature Microbiology demonstrates that, in response to infection, the host immune system forms small, walled-off lesions in the intestines called granulomas. It’s the first time these organized collections of immune cells have been found in the intestines in response to Yersinia infections.
The team went on to show that monocytes, a type of immune cell, sustain these granulomas. Without them, the granulomas deteriorated, allowing the mice to be overtaken by Yersinia.
“Our data reveal a previously unappreciated site where Yersinia can colonize and the immune system is engaged,” says Igor Brodsky, senior author on the work and a professor and chair of pathobiology at Penn Vet. “These granulomas form in order to control the bacterial infection in the intestines. And we show that if they don’t form or fail to be maintained, the bacteria are able to overcome the control of the immune system and cause greater systemic infection.”
The findings have implications for developing new therapies that leverage the host immune system, Brodsky says. A drug that harnessed the power of immune cells to not only keep Yersinia in check but to overcome its defenses, they say, could potentially eliminate the pathogen altogether.
A novel battlefield
Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica share a keen ability to evade immune detection.
“In all three Yersinia infections, a hallmark is that they colonize lymphoid tissues and are able to escape immune control and replicate, cause disease, and spread,” Brodsky says.
Earlier studies had shown that Yersinia prompted the formation of granulomas in the lymph nodes and spleen but had never observed them in the intestines until Daniel Sorobetea, a research fellow in Brodsky’s group, took a closer look at the intestines of mice infected with Y. pseudotuberculosis.
“Because it’s an orally acquired pathogen, we were interested in how the bacteria behaved in the intestines,” Brodsky says. “Daniel made this initial observation that, following Yersinia pseudotuberculosis infection, there were macroscopically visible lesions all along the length of the gut that had never been described before.”
The research team, including Sorobetea and later Rina Matsuda, a doctoral student in the lab, saw that these same lesions were present when mice were infected with Y. enterocolitica, forming within five days after an infection.
A biopsy of the intestinal tissues confirmed that the lesions were a type of granuloma, known as a pyogranuloma, composed of a variety of immune cells, including monocytes and neutrophils, another type of white blood cell that is part of the body's front line in fighting bacteria and viruses.
Granulomas form in other diseases that involve chronic infection, including tuberculosis, for which Y. pseudotuberculosis is named. Somewhat paradoxically, these granulomas—while key in controlling infection by walling off the infectious agent—also sustain a population of the pathogen within those walls.
The team wanted to understand how these granulomas were both formed and maintained, working with mice lacking monocytes as well as animals treated with an antibody that depletes monocytes. In the animals lacking monocytes “these granulomas, with their distinct architecture, wouldn’t form,” Brodsky says.
Instead, a more disorganized and necrotic abscess developed, neutrophils failed to be activated, and the mice were less able to control the invading bacteria. These animals experienced higher levels of bacteria in their intestines and succumbed to their infections.
Groundwork for the future
The researchers believe the monocytes are responsible for recruiting neutrophils to the site of infection and thus launching the formation of the granuloma, helping to control the bacteria. This leading role for monocytes may exist beyond the intestines, the researchers believe.
“We hypothesize that it’s a general role for the monocytes in other tissues as well,” Brodsky says.
But the discoveries also point to the intestines as a key site of engagement between the immune system and Yersinia.
“Previous to this study we knew of Peyer’s patches to be the primary site where the body interacts with the outside environment through the mucosal tissue of the intestines,” says Brodsky. Peyer’s patches are small areas of lymphoid tissue present in the intestines that serve to regulate the microbiome and fend off infection.
In future work, Brodsky and colleagues hope to continue to piece together the mechanism by which monocytes and neutrophils contain the bacteria, an effort they’re pursing in collaboration with Sunny Shin’s lab in the Perelman School of Medicine’s microbiology department.
A deeper understanding of the molecular pathways that regulate this immune response could one day offer inroads into host-directed immune therapies, by which a drug could tip the scales in favor of the host immune system, unleashing its might to fully eradicate the bacteria rather than simply corralling them in granulomas.
“These therapies have caused an explosion of excitement in the cancer field,” Brodsky says, “the idea of reinvigorating the immune system. Conceptually we can also think about how to coax the immune system to be reinvigorated to attack pathogens in these settings of chronic infection as well.”
Igor E. Brodsky is the Robert R. Marshak Professor and chair of the Department of Pathobiology at the University of Pennsylvania School of Veterinary Medicine.
Rina Matsuda is a doctoral student in the Brodsky Laboratory at Penn’s School of Veterinary Medicine.
Daniel Sorobetea is a research fellow in the Brodsky Laboratory at Penn’s School of Veterinary Medicine.
Brodsky, Matsuda, and Sorobetea coauthored the study with Penn Vet’s Stefan T. Peterson, James P. Grayczyk, Indira Rao, Elise Krespan, Matthew Lanza, Charles-Antoine Assenmacher, Daniel P. Beiting, and Enrico Radaelli and University Hospital Regensburg’s Matthias Mack. Brodsky is senior author, and Matsuda and Sorobetea were co-first authors.
The study was supported by the National Institutes of Health (grants AI128530, AI1139102A1, DK123528, AI160741-01, AI141393-2, and AI164655), Burroughs Wellcome Fund, Foundation Blanceflor Postdoctoral Scholarship, Swedish Society for Medical Research, Sweden-America Foundation J. Sigfrid Edström Award, Mark Foundation, and National Science Foundation GRFP Award