 Treating dogs with Krabbe disease, a rare and fatal condition that also affects infants, with a gene therapy targeted to the
Treating dogs with Krabbe disease, a rare and fatal condition that also affects infants, with a gene therapy targeted to the
brain led to remarkable results in a study led by a team from Penn Vet.
In one out of 100,000 infants, a mutation in the GALC gene causes an incurable, always fatal disorder known as infantile Krabbe disease, or globoid cell leukodystrophy. Most children with the condition die before they turn 2.
A parallel condition also naturally affects dogs, who typically show symptoms of the disease at six weeks old and succumb to it within a few months. A study in the Journal of Clinical Investigation (JCI), led by the School of Veterinary Medicine’s Charles Vite, describes an effective gene therapy for Krabbe disease in dogs with lasting impact. Dogs that received the treatment have lived to 4 years of age and beyond with no significant symptoms. The work highlights the potential for a similar treatment approach in children.
“This disease has no good therapy,” says Vite, a professor of neurology and senior author on the new work. “We’ve been looking at this disease in dogs since the 1990s, but it was really the shift over to a new gene therapy vector that gave us a chance to treat it with a big effect on the nervous system.”
Krabbe disease is among a group of conditions known as lysosomal storage diseases, characterized by a buildup of materials in small containers called lysosomes within cells. Normally, the GALC gene encodes an enzyme that breaks down lipids in the body. In Krabbe disease, the mutated GALC causes lipids to build up, resulting in deformed growth of the lipid-containing coating of nerve cells, the myelin sheath, leading to impaired nerve cell signaling. As a result, children with Krabbe disease experience progressive neurological symptoms, including blindness, deafness, and paralysis.
A bone marrow transplant within the first month of life can prevent symptoms from arising in about 30% of infants, but the procedure is exceedingly risky. “A new treatment is really needed,” Vite says.
Krabbe disease was one of the first pediatric genetic diseases for which a parallel inherited disorder was found in dogs. Canines with the condition are part of Penn Vet’s Referral Center for Animal Models of Human Genetic Disease, allowing for the investigation of new therapies.
To blunt the effects of the disease, the researchers knew that getting a healthy version of the GALC gene to the brain was crucial. They were able to make progress in doing so by using a particular vector to deliver GALC gene, the AAV9 vector, which has been used effectively in experimental gene therapies for other neurological diseases and appears the best candidate for FDA approval.
The site of delivery was also important. “We decided we would inject into the spinal fluid via the back of the head, which is the most effective means of getting to the brain,” says Vite.
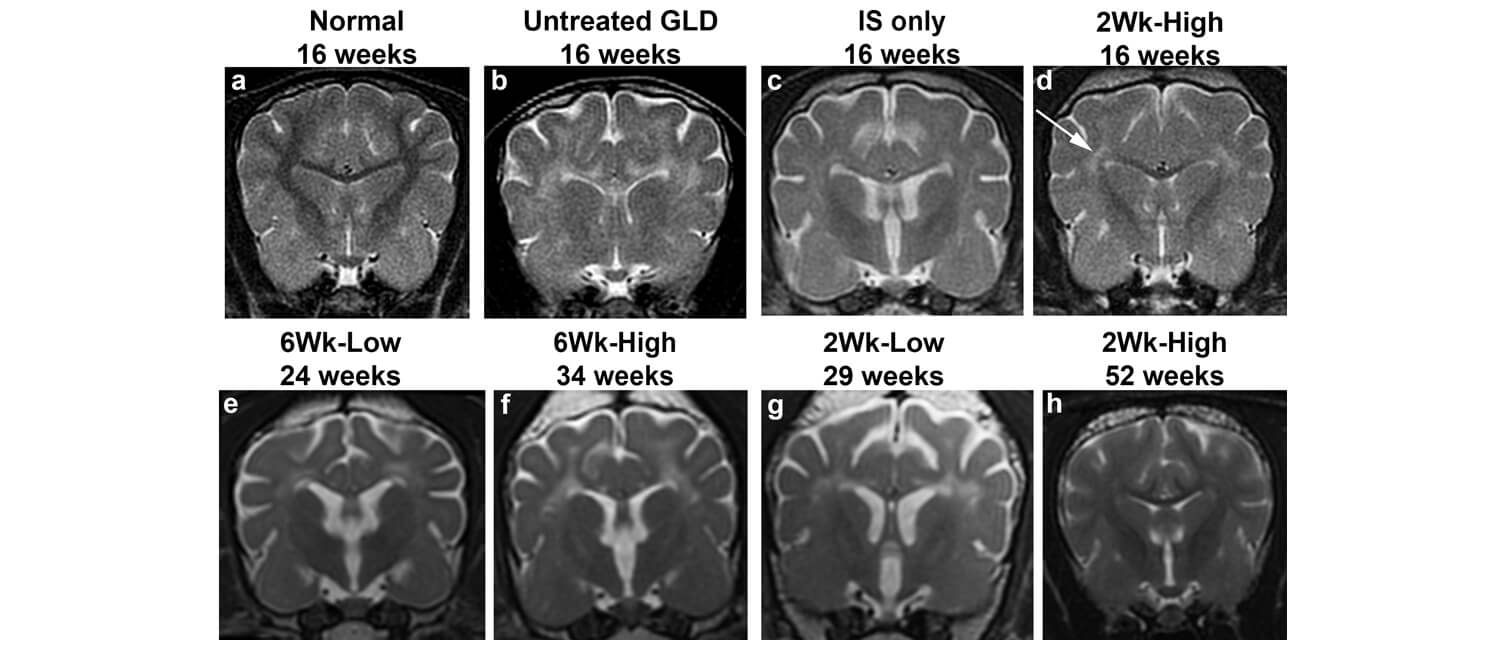
The researchers used both a high and low dose of the gene therapy, administering it to dogs that were two weeks old, before symptoms appear, or six weeks old, after neurological symptoms had begun to emerge.
 (Pre-pandemic image) The work was a collaborative effort by the laboratory of Charles Vite and colleagues. Back row: Ian Hendricks, Petra Werner, Gary Swain, Allison Bradbury, Mark Haskins, Jessica Bagel, and Charles Vite. Front row: Susan Scheerbaum, Magi Casal, Patricia O'Donnell, Caitlyn Molony, Christian Cross, and Vic Stora.
(Pre-pandemic image) The work was a collaborative effort by the laboratory of Charles Vite and colleagues. Back row: Ian Hendricks, Petra Werner, Gary Swain, Allison Bradbury, Mark Haskins, Jessica Bagel, and Charles Vite. Front row: Susan Scheerbaum, Magi Casal, Patricia O'Donnell, Caitlyn Molony, Christian Cross, and Vic Stora.
Vite and colleagues were concerned that simply delivering a normal copy of GALC might not fully alleviate the condition’s symptoms, which result in part due to the buildup of a toxic compound called psychosine from the errant metabolism of the mutant GALC enzyme. But the team was excited by the dramatic results.
Dogs that received the high dose gene therapy before the onset of symptoms not only had healthy myelination in their brains, but the gene therapy also maintained myelination of the peripheral nervous system. “That was a huge surprise for us,” Vite says. “That injecting a gene therapy in the spinal fluid can positively affect both the central and the peripheral nervous system was really exciting.” These dogs have also lived symptom-free for upward of four years.
Even dogs treated after they began to show symptoms lived significantly longer with the therapy than without it. The lower dose of gene therapy, however, resulted in an intermediate form of the disease, underscoring the importance of pinpointing the correct dose when translating the findings to children.
Allison Bradbury, Vite’s former postdoctoral researcher and the lead author on the JCI paper, is now an investigator at Nationwide Children’s Hospital and will be following up on the work to understand how psychosine levels are tamped down by the therapy throughout the body.
Vite’s group, meanwhile, hopes to determine how differences in size and biology between dogs and children can be used to identify an effective dose for both.
And given the effect of the therapy the team observed in the peripheral nerves, Vite is energized by the prospects of such an approach not only in Krabbe disease but in other diseases that involve the peripheral nervous system.
“The hope is to use the model as a method to understand the mechanisms at work in peripheral nerves and how we can target peripheral neuropathies,” says Vite.
Charles Vite is a professor of neurology at the University of Pennsylvania School of Veterinary Medicine.
Allison Bradbury is an assistant professor in the Department of Pediatrics at The Ohio State University and a principal investigator in the Center for Gene Therapy at the Abigail Wexner Research Institute at Nationwide Children’s Hospital. She completed postdoctoral research in the Vite lab.
Vite and Bradbury’s coauthors on the study were Penn Vet’s Jessica H. Bagel, Gary P. Swain, Ian J. Hendricks, Keiko Miyadera, Rebecka S. Hess, Arielle Ostrager, Patricia O’Donnell, and Charles A. Assenmacher; the University of Illinois’ Duc Nguyen and Ernesto R. Bongarzone; the University of Texas Southwestern Medical Center’s Erik A. Lykken and Steven J. Gray; the University of California, San Diego’s Jill Pesayco Salvador and G. Diane Shelton; and Washington University’s Xuntian Jiang, Mark S. Sands, and Daniel S. Ory.
The study was supported by the National Institutes of Health (grants NS096087, OD010939, NS093898, HD096115, and NS065808).