 Bijels, or bicontinuous interfacially jammed emulsion gels, are structured emulsions of oil and water that are kept separated by a layer of nanoparticles. Penn Engineering researchers will develop a way of using them to manufacture mRNA-based therapeutics. (Image: Penn Engineering Today)
Bijels, or bicontinuous interfacially jammed emulsion gels, are structured emulsions of oil and water that are kept separated by a layer of nanoparticles. Penn Engineering researchers will develop a way of using them to manufacture mRNA-based therapeutics. (Image: Penn Engineering Today)
[March 21, 2022; Philadelphia, PA] – Bijels, or bicontinuous interfacially jammed emulsion gels, are structured emulsions of oil and water that are kept separated by a layer of nanoparticles. Penn Engineering researchers will develop a way of using them to manufacture mRNA-based therapeutics. (Image: Penn Engineering Today)
When it emerged in 2019, the SARS-CoV-2 virus quickly revealed itself to be novel in many respects. Able to spread easily, causing a wide variety of symptoms and encircling the globe in a matter of weeks; this microscopic entity reshaped the world forever.
Looked at through a different lens, however, SARS-CoV-2 has a lot in common with pathogens that have come before. It takes advantage of gaps in its hosts’ immune response. It affects men and women differently. And SARS-CoV-2 can be neutralized by a range of vaccines and antiviral compounds that require specialized synthesis, manufacturing, and distribution.
Diving into each of these areas and many more, the pandemic prompted scientists at the University of Pennsylvania to launch new projects, sometimes pivoting from or finding new applications of their research programs. A silver lining of the pandemic may be the resulting discoveries, which stand to benefit humanity long after COVID-19—hopefully—recedes.
Prime among these is the mRNA therapeutic platform Drew Weissman, Katalin Karikó, and others developed, which is now poised to transform vaccination, gene therapy, and other biomedical horizons going forward. And the progress made in antiviral compound assays by researchers like Sara Cherry at the Perelman School of Medicine and Ronald Harty at the School of Veterinary Medicine may prove valuable in this or future pandemics. And these are just the tip of the iceberg.
Two years into this pandemic, Penn Today checked in with a selection of innovative researchers around the University whose work was transformed by the pandemic. These are highlights of their pandemic pivots and the potential impact of their insights.
Viral mimic
Erica Korb, an assistant professor in the Perelman School of Medicine, studies neuroscience and epigenetics—on the surface, not subjects one might expect would overlap with COVID-19.
“But when the pandemic hit and we were all sent home, we were looking for ways to learn something new and keep busy,” she says.
Korb’s group specializes in histone modifications, changes to the proteins around which the genetic material chromatin wraps and twists. Histone modifications can change which portions of DNA are expressed. Other scientific groups had found that some viruses mimic histones to modify their hosts’ gene expression. With lab spaces temporarily closed early in the pandemic, Korb figured one way of “keeping busy” would be to lean into bioinformatics analyses, relying on computational methods. On a bit of a whim, she asked research assistant John Kee to run a computational analysis to see if any of the proteins in SARS-CoV-2 were a match for histone proteins.
“It was a simple thing—so simple nobody else thought to do it,” she says. “I was not expecting to find anything. But he came back to me the next day with a match for one of the most important sequences within the histone proteins. It was a better match than most previously established histone mimics.”
In fact, the virus appeared to mimic two different histone sites, both of which play significant roles in gene expression. The finding suggested that SARS-CoV-2 might be altering the host’s histones and gene expression, perhaps to ease its path to infection.
Partnering with colleagues including Shelley Berger, Susan Weiss, Edward Morrissey, Ben Garcia, and others, the Korb lab has been investigating this feature of the coronavirus ever since. They’re getting ready to publish their discoveries, including an indication that a viral protein has an overall repressive effect on gene expression. In future work, Korb hopes to dig into how this viral capability might relate to how SARS-CoV-2 evades immune defenses, perhaps even pointing to a therapeutic approach to block infection of this or other pathogens.
Gender differences
Early in the pandemic, Montserrat Anguera, an associate professor at the School of Veterinary Medicine, was intrigued by news reports about gender disparities in COVID-19 disease severity: Men tended to get sicker and had higher mortality rates. It sparked a question: Could X chromosome inactivation (XCI) and X-linked gene expression contribute to the sex bias?
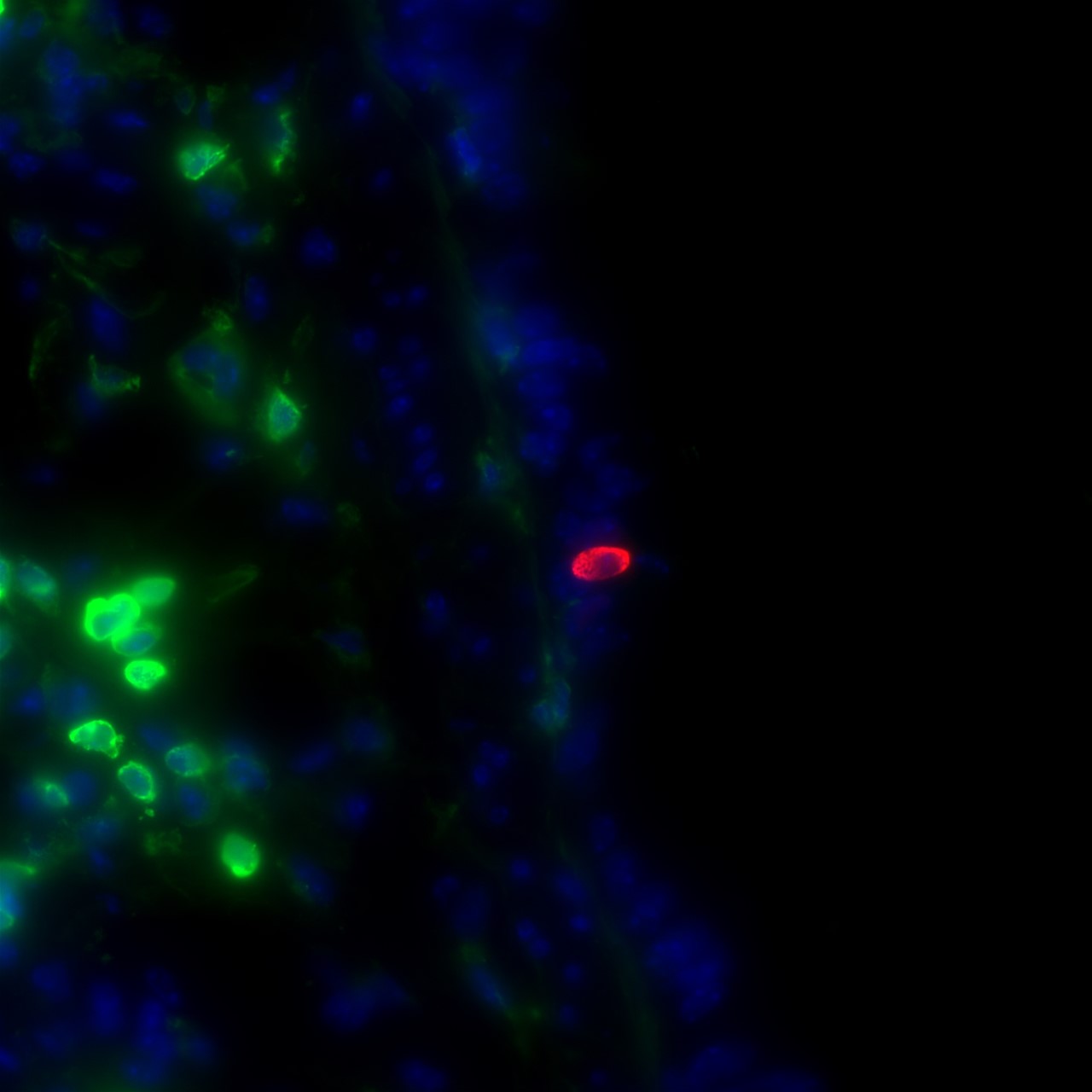 Research in mice by Montserrat Anguera and Andrew Vaughan has examined sex differences in lung cells when it comes to genes relevant to SARS-CoV-2 infection. In a sample from a mouse lung, red labels indicate infection while nearby green labels show the presence of immune cells. (Image courtesy of Andrew Vaughan)
Research in mice by Montserrat Anguera and Andrew Vaughan has examined sex differences in lung cells when it comes to genes relevant to SARS-CoV-2 infection. In a sample from a mouse lung, red labels indicate infection while nearby green labels show the presence of immune cells. (Image courtesy of Andrew Vaughan)
Anguera’s lab studies the phenomenon, by which females with two copies of the X chromosome avoid duplicate gene expression by inhibiting expression from one of the Xs. This inhibition, however, is not always complete, and thus occasionally females may overexpress some X-chromosome-linked genes specifically from the inactive and silent X. Upon realizing that the gene for ACE2, the cellular receptor that SARS-CoV-2 uses to enter cells, is on the X-chromosome, Anguera launched a new path of research into sex differences in coronavirus infection, in collaboration with colleague Andrew Vaughan, whose expertise lies in lung cell regeneration.
“This is just a beautiful example of a collaboration that highlights the strengths of both PIs,” Anguera says. “We’re all about X inactivation and imbalanced gene expression. And Andy is all about these lung cells and how they work.”
Together they developed a mouse with a humanized version of the ACE2 gene to study hormonal and genetic contributions to the pathologies from SARS-Co-V2 infection. Using a novel method whereby they could quantify how much gene expression was occurring from each of the X chromosomes—the “active” as well as the “inactive” X—in lung cells, the researchers found ACE2 was escaping inactivation. And hundreds of other genes were escaping inactivation as well.
“Other people have looked at X-linked gene escape in spleen, brain, and ovary and only seen about 3-7% of expressed X-linked genes escape XCI,” says Anguera. “But with these progenitor lung cells, we’re seeing a ton of escape from the inactive X.”
Despite this escape, the researchers didn’t see more expression of these genes in female mice. They’re looking next to see if that holds true in human cells.
“We’re really excited to continue this deep dive into the genetics of sex differences, and now we have a model system to look at other cell types and how they respond to various viral infections including SARS-CoV-2.”
Viral detection
Despite being more than two years into the pandemic, it’s only relatively recently that effective home tests have been widely available. In the event of a future outbreak of disease, Ping Wang of the Perelman School of Medicine hopes to be way ahead of the game.
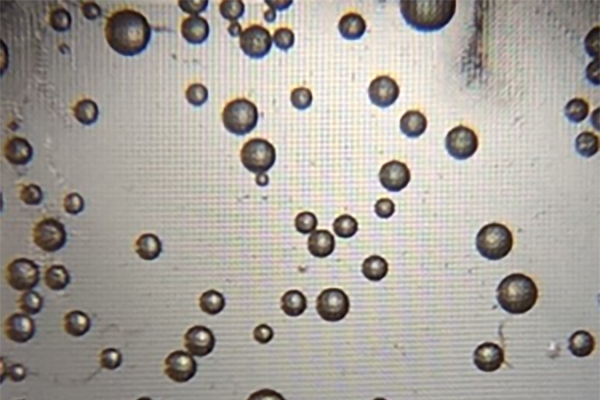
Point-of-care diagnostics was already a focus of Wang’s efforts prior to COVID-19. In 2018, she had developed a proof-of-concept test able to detect protein biomarkers—in that case, prostate specific antigen—with high sensitivity. The approach relied on quantifying the production of microbubbles, which formed during a reaction between hydrogen peroxide, platinum nanoparticles, and the target antigen.
When COVID arrived, it immediately struck Wang that her diagnostic could be used to test for SARS-CoV-2. The microbubbling assay fills what she sees as a gap in current testing: as sensitive as a PCR but with the rapidity of antigen tests.
From that realization, the work to adopt the test platform for SARS-CoV-2 went relatively quickly. But it also made Wang rethink how to market and package the test.
“Before the pandemic we were focusing a lot more using this in physicians’ offices and maybe pharmacy testing,” Wang says. “But I think the pandemic really opened up the need and acceptability of home testing.”
Microbubbles form as a reaction to the bonding of hydrogen peroxide, platinum nanoparticles, and the SARS-CoV-2 antigen, indicating a test result that is positive for COVID-19. (Image: Penn Medicine News)
 The low cost of the microbubbling assay—currently around $3 a test, which may be driven lower with mass production—could also help address inequitable health care access. “Seeing the pandemic expose health disparities really propelled us to think about how we can use our technology as a means to make diagnostics more accessible,” Wang says. She founded the start-up company Instanosis to commercialize the platform.
The low cost of the microbubbling assay—currently around $3 a test, which may be driven lower with mass production—could also help address inequitable health care access. “Seeing the pandemic expose health disparities really propelled us to think about how we can use our technology as a means to make diagnostics more accessible,” Wang says. She founded the start-up company Instanosis to commercialize the platform.
“We didn’t set out to develop this for COVID; we set out really to use it for many other things,” Wang says. “And post-pandemic, our goal is to extend its use. We think the technology is well-poised to be used for many other emerging pathogens or existing pathogens, even multiplex testing of respiratory pathogens and cardiology or neurology biomarkers—many different things that require high-sensitivity biomarker detection.”
Intricacies of immunology
COVID-19 has familiarized many with the importance of antibodies. Made by B cells, their production also relies on T cells, which make B cells better at churning out antibodies that are highly specific to a given pathogen. The type of T cell specialized in this task is known as a T follicular helper (TFH) cell, a centerpiece of Michela Locci’s lab at the Perelman School of Medicine.
Prior to 2020, Locci’s investigations had concentrated the basic biology of TFH cells. But their translational impact was also a focus. “We were already interested in the mRNA vaccine platform, and had been collaborating with [the Perelman School of Medicine’s] Norbert Pardi on it. Even before the pandemic started, I’d never seen a vaccine platform that effective.”
To learn more about the immunological response to mRNA vaccination, Locci wanted to study TFH cells and their role in antibody production in people, not just mice. To do so, she used a technique pioneered by a few other groups in which cells are extracted from the lymph node, where B cells and TFH cells interact. “You essentially poke the lymph node,” she says, “and some cells get stuck in the needle. The analysis of these cells gives you a window into what’s going on at different time points after vaccination.”
Taking this longitudinal approach to tracking development of an immune response “was one of the most exciting changes in the way we approach human immunology during the pandemic,” she says. In collaboration with the medical school’s Ali Naji and Vijay Bhoj, she and colleagues employed the technique in a study published last month in Cell. The research followed a group of kidney transplant recipients, whose immune systems were suppressed, and a group of healthy controls after they both received COVID-19 mRNA vaccines. They found severely impaired B cell and TFH cell responses in the immunosuppressed study participants.
“But in healthy individuals, two doses of mRNA vaccines elicited robust TFH responses, connected with the production of memory B cells and protective antibodies,” Locci says. This connection was not readily appreciable from the study of surrogate populations in blood, whereas it was evident in lymph nodes.
TFH cells play a role in establishing these long-lived B cell responses, and Locci hopes to continue digging into their role in SARS-CoV-2, such as following how these responses may change upon receiving vaccine boosters. But there are myriad applications in other disease and therapeutic avenues, which Locci will explore with a special emphasis on human biology in parallel to mouse biology. “At the end of the day, we particularly care about describing molecular pathways that exist in humans.”
Decentralizing vaccine manufacture
The development of multiple effective vaccines against the SARS-CoV-2 virus in a matter of months was an unprecedented scientific achievement. Yet the distribution of these medical game changers could only proceed as quickly as they could be manufactured in a limited number of facilities. Certain countries and regions waited months to get a supply, dependent on other nations to send them the products.
To Daeyeon Lee and colleagues in the School of Engineering and Applied Science, this obstacle in the supply chain is an engineering problem. In response to a call from the National Science Foundation (NSF) through the Emerging Frontiers in Research and Innovation Program, Lee and others developed a proposal to create a series of hubs where mRNA vaccines could be efficiently manufactured and distributed.
“The call for proposals was looking for ideas in the area of distributing manufacturing of chemicals,” says Lee, a professor and the Evan C. Thompson Term Chair for Excellence in Teaching in the Department of Chemical and Biomolecular Engineering. “When that call came, a bunch of us here at Penn and other institutions thought, Why don’t we apply that idea of distributed manufacturing to the mRNA vaccine?”
Prior to the pandemic, Lee had been working on a system involving simultaneous reaction and separation of different chemicals from reactions, which he could translate to how mRNA molecules are produced. “The idea is, How do we make manufacturing more robust so it can be done in more places?”
Lee, together with collaborator Kathleen Stebe from Penn Engineering and colleagues from the University of Oklahoma, the University of Colorado, and Drexel University, received the NSF grant. That support has led to other funding and opportunities, working in collaboration with Michael Mitchell and David Issadore from Penn Engineering, Penn Medicine’s Drew Weissman, and Infini Fluidics’ Sagar Yadvali to develop their on-demand manufacturing system.
Their vision is a network of research labs, each with the capacity to produce different mRNA therapeutics. The potential is great, as mRNA therapies are showing promise in addressing a host of diseases and disorders, from HIV infection to gene therapy.
“It’s like how the Singh Center, while relatively compact, nevertheless has the capability of fabricating sophisticated, high-tech devices,” Lee says. “We could have these small-scale pharmaceutical mRNA manufacturing centers that can be used during normal times for research purposes but switch over to pharmaceutical production as needed. It’s a way of democratizing the process.”