Research Area #1: Virus-host interactions regulate virus egress and spread
Viruses have developed unique and complex molecular mechanisms to ensure efficient egress of mature virions from infected cells. Our studies are focused on unraveling the intricate roles of both viral and host proteins in this process, and particularly the specific recruitment of host factors to regulate budding of infectious virus. A better understanding of these virus-host interactions and the mechanisms of virus budding not only will provide fundamental insights into the functions of both viral and host proteins, but also will lead to the emergence of novel strategies to inhibit virion egress and spread.
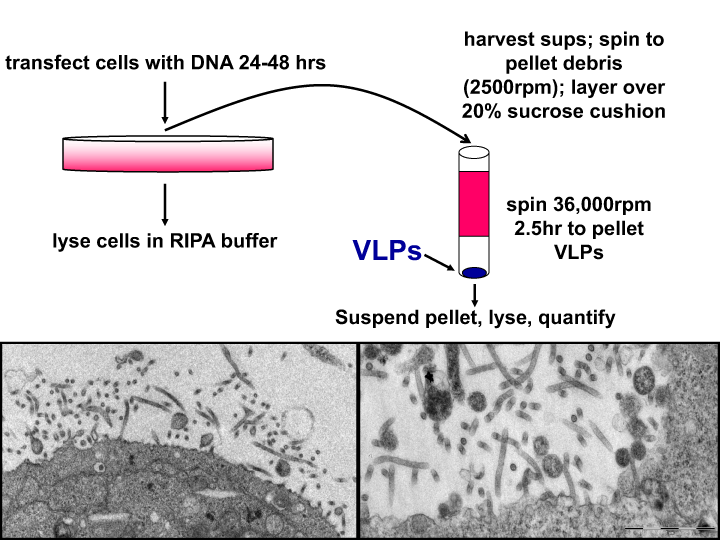 Figure 1:
Figure 1:
Top: Protocol for Virus-Like Particle (VLP) budding assay.
Bottom: Low and high magnification electron micrographs showing budding of long, filamentous Ebola VP40 VLPs from the surface of HEK293T cells. The Ebola VP40 VLPs accurately mimic the morphology and dimensions of live infectious Ebola virus.
The Late (L) budding domain motifs (PTAP and/or PPxY) are highly conserved in the matrix proteins of a wide array of RNA viruses (e.g. filoviruses, arenaviruses, rhabdoviruses, paramyxoviruses, henipaviruses, and retroviruses) and represent attractive and novel targets for the development of therapeutics having broad-spectrum antiviral activity.
Early on we demonstrated that the Ebola virus VP40 protein plays a central and sufficient role in virion assembly and egress, due in part to the presence of overlapping PTAP and PPEY L-domains. We showed that independent expression of VP40 led to the production and egress of virus-like particles (VLPs) that accurately mimic budding of live infectious virus. We used VP40 VLPs and genetically engineered VSV recombinants that expressed WT and mutant L-domain motifs from Ebola virus VP40, to show that efficient budding is dependent on viral L-domain mediated recruitment of host proteins associated with the ESCRT pathway for complete virus-cell fission.
Figure 2: A GFP-Ebola VP40 fusion protein expressed in HEK293T cells shows robust virus-like particle (green) assembly and egress at the plasma membrane. The cytoplasm is stained with HCS CellMask™ Deep Red. This image was generated by the Penn Vet Imaging Core Facility.
The PPxY L-domain motif, conserved in the matrix proteins of Ebola, Marburg, Lassa fever, and VSV, interacts with WW-domains of specific host proteins (e.g. Nedd4) to regulate virus egress. To identify additional WW-domain-bearing host proteins that interact with these viral PPxY motifs, we used viral PPxY-containing peptides to screen an array of 115 mammalian WW-domain-bearing proteins. Results from this screen revealed a plethora of novel PPxY interacting proteins that have both positive and negative effects on VLP and virus budding.
Figure 3: Video highlighting the use of Total Internal Reflection Fluorescence (TIRF) Microscopy to visualize GFP-Ebola VP40 VLPs budding in real-time from the plasma membrane of live HEK293T cells. This video was generated by the PennVet Imaging Core Facility.
For example, we identified BCL2 Associated Athanogene 3 (BAG3) as the first WW-domain-bearing host protein to interact with viral PPxY motifs and negatively regulate egress of Ebola VP40, Marburg VP40, and Lassa Z VLPs. Ongoing projects in the lab are to determine whether additional novel PPxY-interactors identified from this screen play a biologically relevant role in virus assembly and egress.
Research Area #2: Identification and development of host-oriented L-domain inhibitors of virus budding
Based in part on our steady progress in elucidating the molecular aspects and host involvement in virus budding from studies described above, and our long-term goal of developing antivirals, a current major effort in the lab is to identify, develop, and optimize small molecule compounds targeting viral L-domain/host interactions to inhibit virus egress and spread. We postulate that for viruses such as Ebola, administration of such an antiviral therapeutic during an outbreak would inhibit virus dissemination and spread in infected individuals, thus slowing disease progression and allowing the individual’s immune system time to mount a robust response to effectively combat and clear the infection.
Figure 4:
Left: Cartoon diagram of the proposed function of small molecule inhibitors of the viral PTAP-host Tsg101 interaction leading to inhibition of efficient virion egress from the plasma membrane.
Right: Cartoon diagram of the proposed function of small molecule inhibitors of the viral PPxY-host Nedd4 interaction leading to inhibition of efficient virion egress from the plasma membrane.
There remains a vital need for the advancement and development of effective and safe therapeutics against emerging, high priority pathogens such as Ebola, Marburg, and Lassa fever viruses. Since these virus-host interactions represent a common mechanism in a range of emerging RNA viruses, we predict that they represent an Achilles’ heel in the life cycle of these RNA virus pathogens. Toward this end, we have ongoing, fruitful collaborations with medicinal chemists at Fox Chase Chemical Diversity Center in Doylestown, PA, and with virologists at several BSL-4 laboratories which has led to the identification of two successful lead series of PTAP and PPxY budding inhibitors that exhibit on-target, broad-spectrum antiviral activity against a wide array of RNA viruses. For example, our current lead PPxY inhibitors blocked egress of Ebola, Marburg, and Lassa fever VLPs, as well as budding of live infectious viruses in vitro. We continue to employ Structure Activity Relationship (SAR) to identify and test analogs for enhanced potency and low cytotoxicity, as we seek to further transition one or more full-qualified L-domain inhibitors into more detailed IND-directed pharmacokinetic, pharmacodynamic and toxicity studies.
Figure 5: An electron micrograph showing a VSV recombinant containing a mutation in its L-domain resulting in virus particles remaining tethered to the plasma membrane of an infected cell, as they are unable to efficiently undergo fission or “pinch-off” from the cell. Inset – A cartoon diagram depicting the block in L-domain mediated virion egress.
Research Area #3: Innate immune defenses of hemorrhagic fever virus infection
Innate immune responses to virus infection provide a critical first line of defense for the host against the invading pathogen. Understanding the complex interplay between the host innate immune defense mechanisms and counteraction by the filoviruses is crucial for developing novel antiviral strategies, vaccines, and therapeutics. We have been interested in the host innate immune response to filoviruses and have investigated several mechanisms of host innate immune mediated defenses involving host proteins TLR4, SOCS1, SOCS3, and ISG15.
For example, ISG15 is an interferon stimulated gene that has garnered much attention recently due to its broad-range of antiviral activity against a plethora of pathogens including DNA and RNA viruses. We demonstrated for the first time that ISG15 inhibited budding of EBOV VP40 VLPs in a PPxY L-domain dependent manner, and that such inhibition involved impairment of host Nedd4 ligase activity. We continue to investigate whether ISGylation of additional VP40 host interactors can adversely affect VP40 function in budding.
These studies will hopefully reveal new host innate immune defense mechanisms that may regulate the budding processes of several high priority NIAID Category A pathogens. Moreover, this information will be critical to identify strategies (therapies and/or vaccines) designed to tip the scale in favor of the host in the battle between viral pathogens and host innate immune defenses.
